The prevalence of gestational diabetes (GDM) continues to increase in New Zealand, reaching close to 10 per cent in some populations. In 2013, the New Zealand Ministry of Health commissioned the development of clinical practice guidelines on GDM,1 with the aim of providing evidence-based national recommendations. The NZ guidelines differ in several areas, compared to recommendations of the International Association of Diabetes and Pregnancy Study Group (IADPSG),2 which are now supported by the Australasian Diabetes in Pregnancy Society, the World Health Organization and the International Federation of Gynecology and Obstetrics (FIGO). We will focus on where NZ guidelines differ from IADPSG, why they differ and the potential issues for women and babies.
Early screening for undiagnosed pre-existing diabetes mellitus
IADPSG recommends universal early testing, or testing women classified as high-risk, with diagnostic criteria as shown in Table 1. In contrast, the NZ guidelines have opted for universal screening in early pregnancy using only HbA1c. We agree that universal screening is appropriate, due to the increasing prevalence of GDM in our population. HbA1c is the accepted diagnostic test for type 2 diabetes outside of pregnancy3 and can be easily added to routine antenatal blood tests. The NZ guidelines recommend women with HbA1c ≥ 50mmol/mol (6.7%) be referred to a diabetes in pregnancy service for early care, but women with HbA1c 41–49mmol/mol (5.9–6.6%) be offered dietary and lifestyle advice, followed by a 75g oral glucose tolerance test (OGTT) at 24–28 weeks gestation. Both approaches recognise the rising rates of diabetes in the general population and the importance of recognising overt diabetes in early pregnancy. Benefits of this include: decreasing the risk of congenital anomalies in offspring; detection of diabetes complications requiring treatment during pregnancy; prompt restoration of normal glucose levels with treatment; and appropriate treatment of diabetes after pregnancy.
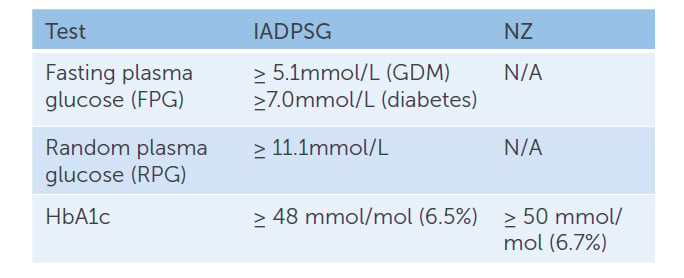
Table 1. IADPSG vs NZ recommendations for early screening for undiagnosed pre-existing diabetes mellitus.
NZ guidelines regarding the management of women with HBA1c 41–49mmol/mol (5.9–6.6%) in early pregnancy have generated considerable local debate. There is little doubt that women with an early pregnancy HbA1c ≥ 50mmol/mol (6.7%) have overt diabetes and should receive care. NZ has chosen a higher cut off (50mmol/mol), compared to other countries (48mmol/L), to align with diagnostic thresholds for diabetes outside of pregnancy. This may lead to under-diagnosis and lends support to the treatment of women with an HbA1c scoring in the high 40s. We would argue that women with an early pregnancy HbA1c 41–49mmol/mol (5.9–6.6%) are a group with increased risk. Providing dietary and lifestyle advice alone, without further support, monitoring or treatment until offering an OGTT at 24 weeks, may mean a missed opportunity to prevent obstetric and neonatal complications.
In a 2014 study4, women with an early HbA1c of 41–46mmol/mol (5.9–6.4%) who were not treated for diabetes, had significantly increased risks of congenital anomaly, pre-eclampsia, shoulder dystocia and perinatal death, compared with women with an HbA1c of less than 41mmol/mol (<5.9%). Some women do not return for their 24–28 week OGTT and some may have a false negative OGTT, which is more likely in obese women5.
Our unit treats women with HbA1c 41–49mmol/mol (5.9–6.6%) in early pregnancy, however, many other units do not and practices differ across NZ. Although these women appear to be at increased risk, it has not yet been established that treating them earlier in pregnancy is effective in reducing this risk. The NZ guidelines opted to wait for more evidence before changing recommendations. Research is ongoing to clarify this issue, with the Pre-diabetes in Pregnancy, Can Early Intervention Improve Outcomes (PINTO) study in NZ and the Treatment of Booking Gestational Diabetes Mellitus (TOBOGM) study in Australia. Proponents for treatment, including us, cite the potential risk reduction of pre-eclampsia, shoulder dystocia and other adverse pregnancy outcomes. Advocates against early treatment cite potential harm from a diagnosis of GDM, such as stigma, pathway shift of obstetric care from low-risk to high-risk and over-medicalisation, as well as the burden of home capillary blood glucose monitoring, dietary modification and clinic visits.
Screening for GDM at 24–28 weeks
The Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study was a landmark international multicentre trial published in the New England Journal of Medicine in 2008.6 It looked at OGTT results for more than 25,000 women and compared these with rates of GDM-related complications. At glucose levels of one standard deviation above the mean, but still below contemporary diagnostic levels for GDM, they found increased odd ratios for all their primary outcomes, with the strongest effect on birth weight and cord blood C-peptide. These relationships were continuous and linear, without a clear threshold. The author’s conclusion was that even ‘mild’ dysglycaemia in pregnancy can have an impact on clinical outcomes and that consideration should be given to lowering the diagnostic thresholds.
IADPSG recommended change to a one-step diagnostic process at 24–28 weeks gestation, with a 75g OGTT and diagnostic thresholds based on the HAPO study results2 (Table 2).
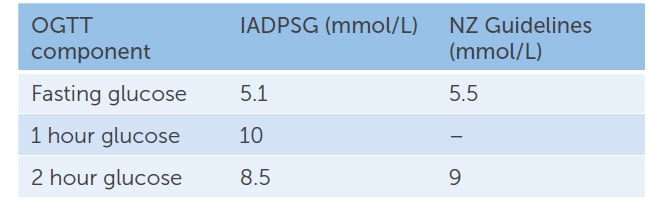
Table 2. IADPSG vs NZ GDM diagnostic glucose cut-offs for 75g OGTT at 24–28 weeks gestation
NZ continues to recommend a two-step process, with a 50g glucose challenge test for women without known risk factors (>11mmol/mol diagnostic, >7.8mmol/L, requiring a follow-up 75g OGTT) and a one-step process with the 75g OGTT for women with risk factors (particularly HbA1c 41–49 mmol/mol 5.9–6.6%). A cost-effectiveness study from 2014 reported the one-step screening strategy would cost NZ$1.38 million more than the two-step screening strategy. Although the authors acknowledged that this approach would miss 12 women with diabetes and 111 women with GDM in their hypothetical cohort of 62,000 women, the additional cost per case detected was high, at NZ$12,460 per case.7
The NZ OGGT thresholds remain higher than other Western countries, with a fasting blood glucose greater than 5.5mmol/L, and 2 h>9.0mmol/L. NZ guideline authors cited concerns that emerging new evidence might suggest a change in thresholds again, reducing consistency of practice in NZ, and that the predicted increase in patient numbers would create unsustainable workforce and financial demands.1
We are now ten years post-HAPO and eight years on from the publication of the IADPSG guidelines. Recent research has been undertaken, comparing outcomes under the old and new criteria. All of the studies show a significant increase in the number of women diagnosed with GDM, with some reporting up to a three-fold increase.8 9 10 However, if this cost is mitigated by improvements in maternal and neonatal outcomes, this increase may not represent an unsustainable burden to the system. The St Carlos Gestational Diabetes Study assessed costs, outcomes and cost-effectiveness before and after the adoption of the IADPSG criteria by a tertiary diabetes clinic in Madrid, Spain.11 The authors found an increase in the rate of GDM (from 10.6% to 35.5%), but also an improvement in pregnancy outcomes, including less gestational hypertension, large for gestational age infants, small for gestational age infants and NICU admissions, as well as lower caesarean rates. Their final analysis showed a cost-benefit of just over €14,000 for each 100 women screened with the new criteria.
In NZ, the Gestational Diabetes Mellitus Study of Diagnostic Thresholds (GEMS) is looking at outcomes by comparing the current NZ and IADPSG criteria. We feel that NZ policy makers need to look at the whole picture of care using the St Carlos study approach. Our concern is that, while NZ waits for more evidence, a generation of at-risk women and babies will have missed out on interventions that could improve their health now and in the future.
If other studies confirm the results of the St Carlos study, then there will be a strong case for NZ to move to the IADPSG criteria. While some fear this may put a strain on already stretched services, in our opinion, the increased number of women diagnosed with GDM could be offset by improvements in outcomes and overall reduction in costs. Current models of care could be reviewed to meet the increased demand, triaging lower risk women to be managed in less costly community settings. What is required is not an approach that ignores some women with GDM, but one which creates innovative ways of managing women appropriately in different settings. In the interim, we encourage all NZ clinicians to offer and support participation in the PINTO and GEMS trials.
We remind NZ midwives, obstetricians and physicians to be aware that women in NZ with pre-pregnancy pre-diabetes and those currently diagnosed with GDM are higher risk compared to international populations. Careful monitoring during and after pregnancy is required.
References
- NZ Ministry of Health. Screening, Diagnosis and Management of Gestational Diabetes in New Zealand: A clinical practice guideline. Wellington 2014.
- International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care. 2010;33(3):676.
- NZ Society for the Study of Diabetes. NZSSD Position statement on the diagnosis of, and screening for, type 2 diabetes. 2011.
- Hughes RCE, Moore MP, Gullam JE, Mohamed K, Rowan J. An early pregnancy HbA1c ≥5.9% (41 mmol/mol) is optimal for detecting diabetes and identifies women at increased risk of adverse pregnancy outcomes. Diabetes Care. 2014;37(11):2953.
- Rowan JA, Budden A, Sadler LC. Women with a nondiagnostic 75 g glucose tolerance test but elevated HbA1c in pregnancy: An additional group of women with gestational diabetes. ANZJOG. 2014;54(2):177-80.
- Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. NJEM. 2008;358(19):1991-2002.
- Coop CE, R. Brown, J. Farquhar, C. Cost-effectiveness of
the New Zealand diabetes in pregnancy guideline screening recommendations. BMJ. 2015;5(6). - Tan HLE, Luu J, Caswell A, Holliday E, Attia J, Acharya S. Impact of new International Association of Diabetes and Pregnancy Study Groups (IADPSG) diagnostic criteria on perinatal outcomes in a regional tertiary hospital in New South Wales, Australia. Diabetes Research and Clinical Practice. 2017;134:191-8.
- Ekeroma AJ, Chandran GS, McCowan L, Ansell D, Eagleton C, Kenealy T. Impact of using the international association of diabetes and pregnancy study groups criteria in South Auckland: prevalence, interventions and outcomes. ANZJOG. 2015;55(1):34-41.
- Wendland EM, Torloni MR, Falavigna M, Trujillo J, Dode MA, Campos MA, et al. Gestational diabetes and pregnancy outcomes – a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy and Childbirth. 2012;12(1):23.
- Duran A, Sáenz S, Torrejón MJ, Bordiú E, del Valle L, Galindo M, et al. Introduction of IADPSG criteria for the screening and diagnosis of gestational diabetes mellitus results in improved pregnancy outcomes at a lower cost in a large cohort of pregnant women: The St. Carlos Gestational Diabetes Study. Diabetes Care. 2014;37(9):2442.






The correct name for the society is the Australasian Diabetes in Pregnancy Society. It specifically includes New Zealand.
Thank you very much for bringing that to our attention – it has now been amended. We apologise for the error.