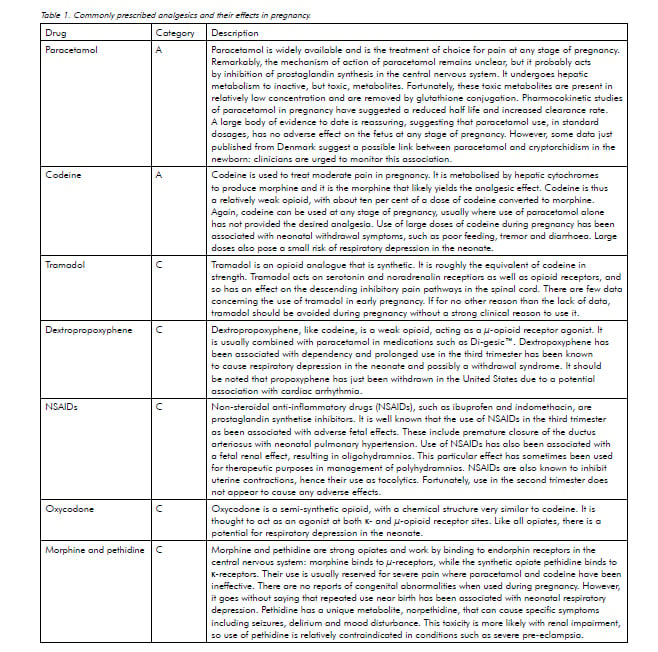
Symptoms of pain, such as headache and musculoskeletal discomfort, are common during pregnancy. Women and their doctors are often nervous about the use of analgesics during pregnancy and frequently seek advice about their safety.
The efficacy of most drugs is directly related to the concentration of the drug in the plasma. In turn, the plasma concentration of drugs is ultimately determined by the absorption of the drug, its distribution through the body and eventual elimination. These considerations thus influence the dosing (both amount and frequency of doses) and the route of administration. The aim of drug use is to achieve an effective plasma concentration, enough to achieve the desired therapeutic effect, yet reduce the potential for any toxicities as much as possible.
When drugs are taken orally, passive diffusion across the small intestinal wall is the primary route of absorption. Once absorbed, the drug will pass through the hepatic microcirculation in the first instance. Some drugs are strongly affected by this ‘first pass’ effect. Once into the systemic circulation, drugs are then distributed through the intravascular, interstitial and intracellular spaces. Drugs with a large molecular weight, such as heparin, are confined to the intravascular space, whereas lipophilic drugs will be widely distributed through all of the compartments.
As the drugs circulate, they will be subject to elimination. Polar drugs are commonly eliminated through the kidneys, whereas lipophilic drugs mostly undergo metabolism in the liver and the metabolites are cleared by the kidney on subsequent passes. Most drugs have a relatively constant rate of clearance that is not dependent upon the plasma concentration. This is a process known as linear kinetics and this allows accurate estimation of plasma concentrations of a drug to be made for the time after administration. However, notable exceptions include alcohol (if you consider alcohol a drug!) and some anticonvulsants.
For most drugs, those that follow linear kinetics, the plasma concentration will rise after administration, to reach a peak and then fall predictably. The time for the plasma concentration to fall by 50 per cent is called the ‘half life’. Drugs that are administered by a regular dosage schedule will usually rise over about three half-lives to reach a steady state concentration. Where it is important to reach a therapeutic plasma concentration promptly, a loading dose of the medication may be given.
Pregnancy imposes physiological changes that begin very early, during the first trimester. Gastric emptying and small intestine motility are delayed in pregnancy. This is likely to be the effect of progesterone. As well, changes in gastric pH and mucus production can alter ionisation of weak acids and reduce their absorption. This is not commonly a problem with repeated dosage schedules, but can certainly affect single-dose drugs such as analgesics or anti-nauseants. To make matters worse, vomiting and anorexia often lead to loss of part or all of a drug dose, or to postponement of doses.
The well-known increase in intravascular volume contributes to a total increase in body fluid of up to ten litres in some women. The effect can be complex with changes in protein binding, since haemodilution can reduce plasma albumen concentrations. To further complicate things, steroid hormones (present in high concentration in some pregnant women) may displace drugs from binding sites on proteins. This can result in increased concentrations of some drugs that are normally bound to proteins. Although free drugs are more promptly metabolised and excreted, meaning that clinical effects are not necessarily altered, monitoring of drug concentrations can be difficult and misleading.
Pregnancy increases the activity of cytochromes in the liver, leading to an increased rate of metabolism and elimination of drugs. At the same time, steroid hormones can inhibit elimination through other isoenzyme systems. Blood flow through the kidneys increases, increasing by up to two thirds, with a resultant increase in glomerular filtration rates. Drugs – such as some antibiotics that are excreted essentially unchanged – are thus removed much more quickly and have lower steady state concentrations.
Diffusion across the maternal-fetal interface in the placenta favours lipophilic drugs, but this is rate limited by the blood flow to the placenta. Large drug molecules, such as heparin, and strongly protein-bound drugs, such as insulin, cannot cross the placenta. Analgesics readily cross the placenta to the fetus. Cells in the fetal liver and placental tissues can metabolise drugs, but fetal cells are immature and the ductus venosus directs much incoming fetal blood past the liver directly to the fetal heart and brain.
The fetus eliminates drugs mostly by diffusion back across the placenta. Since many drug metabolites are polar, the metabolites will build up in the fetal compartments. Elimination through the kidneys sees these accumulate in the amniotic fluid and thus may be re-ingested by the fetus.
In summary, pharmacokinetic changes in pregnancy are complex. Furthermore, there is an understandable hesitancy by researchers and pharmacology companies to undertake the relevant drug studies in pregnant women. The data that do exist are usually from small studies and do not take into account the differences at different gestations.
The pharmacology and pharmacokinetics of the common analgesics are little-studied in pregnancy. Fortunately, most have a good safety profile if used for brief periods in healthy pregnant women. There are few incentives for pharmaceutical companies to undertake large scale studies of drugs in pregnancy so don’t hold your breath for more information to arrive any time soon.
References
Dawes M, Chowienczyk PJ. Pharmacokinetics in pregnancy. Best Pract Res Clin Obstet Gynaecol 2001; 15: 819-26.
Kristensen et al. Intrauterine exposure to mild analgesics is a risk factor for development of male reproductive disorders in human and rat. Hum Reprod 2011;26(1)235-244.
Vickers M, Brackley K. Drugs in pregnancy. Current Obstet Gynaecol 2002; 12: 131-7.
Weiner CP, Buhimschi C, Swaan P. Drug prescribing challenges during pregnancy. Current Obstet Gynaecol 2005; 15: 157-65.






Leave a Reply