I have received a few raised eyebrows from family and friends about my writing an article on the topic of ‘inflatables’. I promise, you will not be disappointed. This article outlines three different inflatable devices that are available to assist us as obstetricians in managing a diverse range of familiar scenarios.
Fetal Pillow
The latest inflatable on the obstetric scene,1 the Fetal Pillow has been designed to elevate the fetal head vaginally and is particularly useful for second-stage caesarean sections. The increased intraoperative morbidity of a full-dilatation caesarean section, particularly uterine extensions into the broad ligament or vagina with subsequent postpartum haemorrhage (PPH), is thought to be caused by the increased manipulation required to deliver a deeply impacted head with caput and moulding through a thin and oedematous lower segment.2 The premise of this inflatable is to achieve an atraumatic delivery of the vertex from deep within the maternal pelvis without recourse to established methods for difficult head delivery, such as elevation of the vertex vaginally by an assistant’s hand, use of uterine tocolytics or breech extraction via an extended uterine incision.
The Fetal Pillow is a disposable soft silicone balloon that is inserted preoperatively behind the fetal vertex, similar to the placement of a ventouse cup for an occipito-posterior position (see Figure 1). The balloon inflates in only an upward direction when the tubing is injected with 180ml of sterile saline with its base plate resting on the anococcygeal ligament. After delivery, the balloon is deflated via the two-way tap and the device is removed from the vagina with traction. Given the need for the Fetal Pillow to be positioned prior to caesarean section, this device is ideally suited to cases where there has been an examination in theatre to assess suitability for an instrumental vaginal delivery or a prior attempt made to achieve an instrumental vaginal delivery.
The Fetal Pillow is an innovative device, but there currently remains a paucity of evidence. The National Institute for Health and Care Excellence (NICE) has recommended that the device is only used within an audit and research framework owing to the inadequate quantity and quality of current evidence.3 A number of centres in the UK, as well as in India and Australia, have reported their positive anecdotal experience with the use of the Fetal Pillow. The initial Australian experience was evaluated in a retrospective cohort study of all full-dilatation caesareans over a 13-month period.4 Of 265 full-dilatation caesarean sections, the Fetal Pillow was used in 35 cases while elevation of the fetal head vaginally by an assistant was used in 29 cases, with a comparison of maternal and neonatal outcomes between both groups. There were no differences in the rates of uterine extensions, PPH, blood transfusion, neonatal resuscitation or admission to neonatal intensive care. There was a significantly higher cord arterial pH in the Fetal Pillow group (pH 7.24 versus 7.19, p<0.019).
The best available evidence for the Fetal Pillow comes from a prospective non-randomised comparative study undertaken in India.5 This compared the maternal and fetal complications in full-dilatation caesarean sections between women who had a Fetal Pillow inserted prior to their caesarean (n=50) against unmatched historical controls (n=124). Women in the Fetal Pillow group had a lower incidence of extensions (four per cent versus 15 per cent, p=0.03), shorter operating time (31.8 minutes versus 52.1 minutes, p<0.001), shorter length of hospital stay (4.1 versus 6.4 days, p<0.001). The rate of PPH above 1L was lower in the Fetal Pillow group (two per cent versus eight per cent) although this did not reach statistical significance (p=0.18).
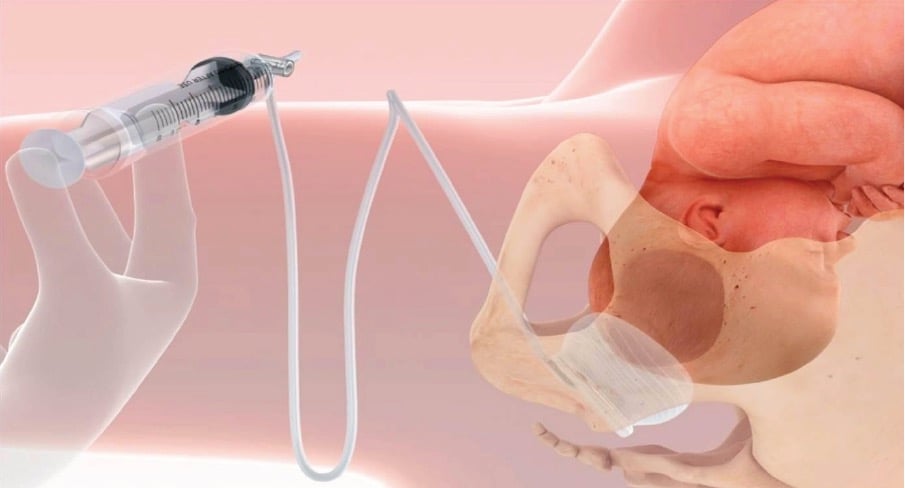
Figure 1. Positioning and inflation of the Fetal Pillow.
Intrauterine inflatable balloons
The modern management of PPH has adopted the use of inflatable intrauterine balloon devices, replacing the former practice of uterine packing with sterile gauze, with its potential disadvantages of infection and trauma. The stepwise approach to atonic PPH involves the use of uterotonic medications, uterine rubbing and bimanual compression. If these prove ineffective, patient management becomes surgically orientated, following the exclusion of retained products, genital tract trauma and coagulopathy. The intrauterine balloon is a less-invasive surgical intervention compared to uterine compression sutures (such as B-Lynch sutures), internal iliac artery ligation or peripartum hysterectomy, and avoids the serious complications of invasive surgery such as additional blood loss, visceral damage, long postoperative recovery and impact on fertility. Furthermore, its simplicity of use makes it an invaluable tool in the management of PPH in units with junior medical staff or staff inexperienced to perform invasive surgery, or where there is no access to an interventional radiology service for uterine artery embolisation.
The mechanism of action of the intrauterine balloon has long been assumed to be effected by an inward-to-outward pressure that occludes the venous sinuses within the uterine cavity. However, more recent evidence suggests that intrauterine balloons induce uterine contractility.6
The Bakri device, originally called the Surgical Obstetric Silicone (SOS) balloon was designed specifically as an intrauterine device for controlling obstetric bleeding. There are a number of alternative intrauterine balloon devices that have been ‘borrowed’ from other specialties and used in PPH management where the Bakri balloon is not available or too expensive.7 These include the Rusch balloon (a urological device), the Sengstaken- Blakemore tube (a device used to control bleeding oesophageal varices), single and multiple Foley urinary catheters and the condom catheter, which is a low-cost option using a condom tied to the tip of a Foley’s catheter (see Figure 2).
The original intrauterine tamponade technique described by Bakri involved the use of 5–10 Foley urinary catheters tied together and placed in the lower segment at the time of caesarean section for controlling bleeding from six cases of placenta praevia.8 Ten years later, Bakri custom-designed an intrauterine balloon device for controlling PPH associated with placenta praevia and accreta.9 The Bakri balloon is now commonly used to control atonic PPH following a vaginal delivery, with the benefit of avoidance of a laparotomy if the tamponade is effective. In some clinical settings, the Bakri balloon serves as a holding measure to enable resuscitation of the women, obtaining cross-matched blood and senior help.
The Bakri balloon is 58cm long and is made of silicone, making it suitable for use in patients with a latex allergy. The recommended volume of inflation from the manufacturer (Cook Medical, USA) is 500ml. Higher or lower volumes may be used to achieve the tamponade effect, although the balloon has a maximum capacity of 800ml. Ultrasound can be used to visualise the correct placement of the balloon, particularly in women at high risk of perforation. The Bakri balloon has a large bore channel that allows free drainage of blood from the uterine cavity and therefore immediate recognition of ongoing bleeding.
Insertion of the Bakri balloon vaginally involves using several sponge holders to hold the cervix and then threading the deflated balloon catheter through to the fundus, either digitally or using an a sponge holder. There is a double lumen shaft, one has a two-way tap for inflation of the balloon with sterile water and the other, for drainage, is attached to a collecting bag.
At caesarean section, the balloon can be introduced abdominally and the distal end of the balloon shaft passed through the cervical opening for an assistant to pull the end out vaginally. In this scenario, balloon inflation occurs after the uterine incision is closed, with potential risk of balloon puncture during uterine suturing. Alternatively, the uterus is closed and the balloon is inserted vaginally, with completion of laparotomy closure once uterine tamponade is confirmed. Whichever method is used for insertion, the original technique described by Bakri involves packing the vagina with gauze around the balloon shaft to separate the intrauterine balloon from the vagina, thereby achieving maximum tamponade effect.10 However, vaginal packing may only be required in cases with a dilated cervix to prevent displacement of the balloon into the vagina. Most operators use a prophylactic antibiotic in theatre in view of contamination of the uterine cavity from the vaginal environment and some operators continue antibiotics for the duration of the balloon placement.
Following deflation of the balloon, the Bakri device is easily removed vaginally. The balloon can be deflated completely or sequentially, usually within a timeframe of 12–48 hours, depending on the severity of the PPH and the availability of senior staff if there is continued bleeding.
The Bakri balloon has an excellent overall success rate of 91 per cent from case reports, retrospective and prospective studies.11 Failures of the Bakri balloon include unrecognised genital tract trauma, the development of coagulopathy or displacement of the balloon through an open cervix. It has few contraindications, the main one being active uterine infection. Several complications have been reported with the use of Bakri, including uterine perforation during insertion, uterine rupture from uterine over-distension, postoperative pain, endometritis, ulceration and pressure necrosis in the uterus or vagina and intrauterine adhesions.12 The use of combined procedures, such as uterine compression sutures with a Bakri balloon, increases the incidence of complications.
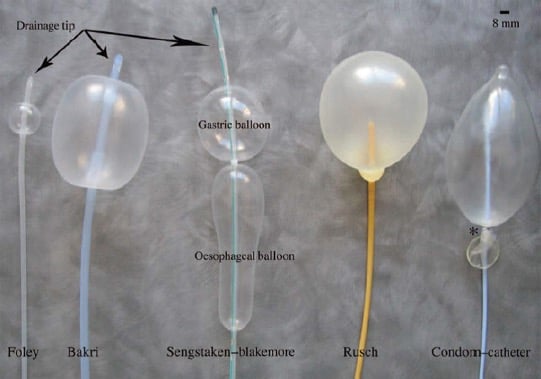
Figure 2. The range of intrauterine balloon devices.
Balloon catheters for IOL
Finally, there is increasing use of an inflatable balloon device for the most common of all obstetric interventions, induction of labour (IOL). This might be construed as a novel method for cervical ripening without the need for pharmacological agents. However, mechanical methods of IOL are among the oldest methods of induction prior to the development of prostaglandins. Although there are single- and double-chamber balloon catheters that have been specially designed for IOL, a Foley’s urinary catheter can also be employed.
There are a number of advantages to the use of a balloon catheter for IOL, including its low cost, no requirement for refrigerated storage, the option for less frequent fetal monitoring, avoidance of the side effects associated with the use of prostaglandins as well as the potential for outpatient management.
Insertion of a balloon catheter for IOL is a simple procedure performed in a lithotomy position without the need for analgesia. In some induction units, midwives are primarily responsible for balloon insertions. A preliminary cervical assessment to assess the Bishop’s score is performed to redirect those women with a suitably favourable cervix to delivery unit for an amniotomy. A speculum is inserted into the vagina to visualise the cervix, which is cleansed with an antiseptic. The balloon catheter is inserted through the internal cervical os under vision using a sponge holder to assist with placement. The balloon is inflated with 50ml of sterile water and the end of catheter spigoted. The catheter is gently withdrawn until the resistance of the internal os is felt and is then secured to the woman’s inner thigh with tape. The woman can mobilise freely, toilet and shower with the catheter in place. A short cardiotocograph is performed after the balloon insertion and as required for subsequent fetal monitoring. If spontaneous rupture of membranes occurs, the balloon is deflated and removed. If the catheter falls out, a cervical assessment is performed to assess favourability for amniotomy. Otherwise, the balloon is deflated and removed 24 hours after insertion to plan the next stage of the IOL process.
Mechanical methods for labour induction have been the subject of a recent Cochrane review from 2012.13 There were 23 studies including more than 3000 women that compared the balloon catheter method with any prostaglandin. Women in the balloon catheter group had a reduced risk of hyperstimulation with fetal heart rate changes compared to the use of prostaglandins (0.4 per cent versus three per cent, relative risk 0.19, 95 per cent confidence interval 0.08–0.43) without a significant difference in risk of caesarean section between both groups (27 per cent versus 25 per cent, relative risk 1.01, 95 per cent confidence interval 0.90–1.13). Women in the balloon catheter group had a non-statistically significant increased risk of not achieving vaginal delivery within 24 hours compared to women in the prostaglandin group (48 per cent versus 38 per cent, relative risk 1.26, 95 per cent confidence interval 0.94–1.68). Interestingly, there was greater need for the use of oxytocin in the subsequent course of labour for women in the balloon catheter group (75 per cent versus 50 per cent, relative risk 1.51, 95 per cent confidence interval 1.15–1.97). Serious maternal and neonatal morbidity rates were not routinely reported in the studies but were infrequent and did not differ between the groups.
Given the evidence for a reduced rate of uterine hyperstimulation with balloon catheters, they have been rapidly adopted as the method of choice for IOL in those clinical situations in which there is a particular indication to avoid hyperstimulation, commonly women with a prior uterine scar and where there are concerns regarding fetal wellbeing, such as intrauterine growth restriction or abnormal Dopplers.
There are theoretical concerns regarding the risk of infection with the use of inflatable catheters for IOL, particularly as they are retained in the cervix for 24 hours. One systematic review from 2008 specifically addressed maternal and neonatal infectious morbidity.14 Compared with women using pharmacological agents, women undergoing induction with mechanical methods had a higher likelihood of infectious morbidity (odds ratio 1.38) and a higher risk of neonatal infectious morbidity (odds ratio 2.03).
The most recent development in the use of balloon catheters for IOL has been in investigating their suitability for outpatient management. A recent Australian randomised trial of outpatient Foley catheter (n=50) versus inpatient prostaglandin gel (n=51) for IOL has reported no differences in safety outcomes including Apgar scores, neonatal admission rates and cord gas values.15 Although the outpatient catheter group had a shorter hospital stay prior to birth, with less pain and more sleep during cervical preparation, they were more likely to require oxytocin and there were no statistically significant reductions in total inpatient stay.
This article has outlined the practical use of three very different inflatable balloon devices for the management of common obstetric scenarios. Whether or not you have previously used some or all of these inflatables, I hope to have inflated your knowledge and confidence in using them!
References
- Fetal Pillow. www.fetalpillow.com .
- Murphy DJ, Liebling RE, Verity L, Swingler R, Patel R. Early maternal and neonatal morbidity associated with operative delivery in the second stage of labour: a cohort study. Lancet. 2001;358:1203-1207.
- National Institute for Health and Care Excellence. Insertion of a balloon device to disimpact an engaged fetal head before an emergency caesarean section. Interventional procedure guideline. 2014. Available at nice.org.uk/guidance/ipg515 .
- Safa H, Beckmann M, Wight K. The use of the fetal pillow to deliver the fetal head at caesarean section at full dilatation. BJOG. 2015; 122 (Suppl. 1): 211.
- Seal SL, Dey A, Barman SC, Kamilya G, Mukherji J. Does elevating the fetal head prior to delivery using the fetal pillow reduce maternal and fetal complications in a full dilatation caesarean section? A prospective study with historical controls. Journal of Obstetrics and Gynaecology, 2014;34:241-244.
- Marasinghe JP, Du Plessis J, Epitawela D, Umstad MP. Management of postpartum haemorrhage with uterine balloon tamponade: The way forward. ANZJOG, 2015;55:315-317.
- Georgiou C. Balloon tamponade in the management of postpartum haemorrhage: a review. BJOG. 2009;116:748-757.
- Bakri YN. Uterine tamponade-drain for obstetrical haemorrhage secondary to placenta previa-accreta. International Journal of Gynaecology and Obstetrics. 1992;37:302-3.
- Bakri YN, Amri A, Jabber FA. Tamponadeballoon for obstetrical bleeding. International Journal of Gynaecology and Obstetrics. 2001;74:139-42.
- Bakri YN, Amri A, Jabber FA. Tamponadeballoon for obstetrical bleeding. International Journal of Gynaecology and Obstetrics. 2001;74:139-42.
- Georgiou C. Balloon tamponade in the management of postpartum haemorrhage: a review. BJOG. 2009;116:748-757.
- Georgiou C. Balloon tamponade in the management of postpartum haemorrhage: a review. BJOG. 2009;116:748-757.
- Jozwiak M, Bloemenkamp KWM, Kelly AJ, Mol BWJ, Irion O, Boulvain M. Mechanical methods for induction of labour. Cochrane Database of Systematic Reviews 2012, Issue 3.
- Heinemann J, Gillen G, Sanchez-Ramos L,Kaunitz AM. Do mechanical methods of cervical ripening increase infectious morbidity? A systematic review. American Journal of Obstetrics and Gynaecology. 2008;199(2):177-187.
- Henry A, Madan A, Reid R, Tracey SK, Austin K, Welsh A. Outpatient Foley catheter versus inpatient prostaglandin E2 gel for induction of labour: a randomised trial. BMC Pregnancy and Childbirth. 2003;13:25.





Leave a Reply