It’s time to prioritise iron optimisation in pregnancy
Dear Editor,
We read with great concern Dr Barton Smith’s article in O&G Magazine, 2022, vol.24, no. 1. ‘Normal serum-ferritin in pregnancy: less is more’. We wish to dispute some alarming claims made by the author thus highlighting the importance of correcting obstetric iron deficiency for both mother and baby.
Dr Smith argues that the development of iron deficiency, which he mislabels hypoferritinaemia, ‘serves two logical protective purposes’ namely protection against sepsis and miscarriage. Dr Smith fails to acknowledge the complex interplay between iron and immunity negating to mention the important role iron plays in humoral immunity including neutrophil, macrophage, and T cell function.1 Secondly, whilst we agree that obstetric complications are increased in iron overloaded states, it cannot be extrapolated that the correction of iron deficiency is harmful. This is analogous to tolerating maternal hypothyroidism due to the well-recognised adverse obstetric outcomes associated with hyperthyroidism.
The article makes no mention of the breadth of evidence supporting the negative effects of obstetric iron deficiency. Iron plays an essential role in cellular respiration and mitochondrial function and is a cofactor for the synthesis of the neurotransmitters dopamine and serotonin.2 These non-erythropoietic roles explain why individuals with iron deficiency can be symptomatic even in the absence of anaemia with manifestations including fatigue, poor cognition, reduced physical performance and mood disturbances.3 4 Small randomised trials have shown that correction of iron deficiency improves the ability to perform simple and complex cognitive tasks whereas correction of anaemia merely improves the speed in which these tasks are performed.5 Specific to the obstetric population is iron’s impact on maternal infant interactions and link to postpartum depression.6 7
Whilst studies on the correction of obstetric iron deficiency fail to prioritise quality of life scores as outcome measures, objective measures of anaemia at birth and risk of blood transfusion are reduced,8 and whilst some may take the decision to transfuse lightly, it is not without risk including a 10% risk of alloimmunisation9 which may negatively impact future pregnancies in regards to the risk of haemolytic disease of the fetus and newborn (HDFN).
There is evidence to support that neonates born to iron deficient mothers can develop iron deficiency with or without anaemia,10 and in observational studies this has been linked to inferior neurocognitive performance at five and 10 years namely in areas of language ability and fine-motor skills.11 Additionally, lasting behavioural issues have been seen at five years despite correction of this iron deficiency in infancy.12
Finally, whilst we acknowledge the concern in the obstetric community about the use of intravenous (IV) iron, we believe that ignoring the issue is not the solution; rather, we advocate for a proactive approach to iron optimisation in obstetrics with early identification of iron deficiency prior to the onset of anaemia. This also facilitates the greater use of oral iron through smaller doses or alternate day dosing thereby reducing the reliance on IV iron. Waiting for the woman to ask for iron replacement, as Dr Smith states, is unacceptable.
Iron optimisation in women working group:
- Dr Lisa Clarke
Transfusion Policy and Education, Australian Red Cross Lifeblood, NSW, Australia
Haematology, Sydney Adventist Hospital, NSW, Australia - Dr Kylie King
Department of Haematology, Wollongong Hospital, NSW, Australia
Graduate School of Medicine University of Wollongong, NSW, Australia - Dr Catherine Tang
Department of Haematology, Gosford Hospital, NSW, Australia
School of Medicine and Public Health, University of Newcastle, NSW Australia - Dr Lynn Townsend
Ultrasound Care Australia
School of Women’s and Children’s Health, UNSW Medicine & Health, NSW, Australia - Dr Talat Uppal
Women’s Health Road, NSW, Australia
Department of Obstetrics and Gynaecology, Northern Beaches Hospital, NSW, Australia - Dr Nada Hamad
Department of Haematology, St Vincent’s Hospital Sydney, Australia
School of Clinical Medicine, UNSW Medicine & Health, NSW, Australia
School of Medicine, University of Notre Dame, NSW, Australia
Right of reply
Dear Editor,
Thank you for inviting me to reply to ‘It’s time to prioritise iron optimisation in pregnancy’.
Dr Clarke et al assert a failure to acknowledge ‘the important role iron plays in humoral immunity including neutrophil, macrophage and T-cell function’. In doing so, they incorrectly state T-cell function is a component of humoral immunity, when it is in fact cellular. More importantly, there is no evidence that intracellular immunity is deficient in iron in response to the normal pregnancy iron debt, or that gestational iron enhances immunity. There is, however, evidence that intravenous (IV) iron therapy worsens infections,13 which is one reason the statement ‘it cannot be extrapolated that the correction of iron deficiency is harmful’ is incorrect. Other harms of gestational iron therapy include permanent staining,14 hypophosphataemia,15 16 17 financial cost,18 myalgia,19 fishbane reaction,20 anaphylaxis,21 death,22 bloating, constipation, haemorrhoids, and anal fissures.
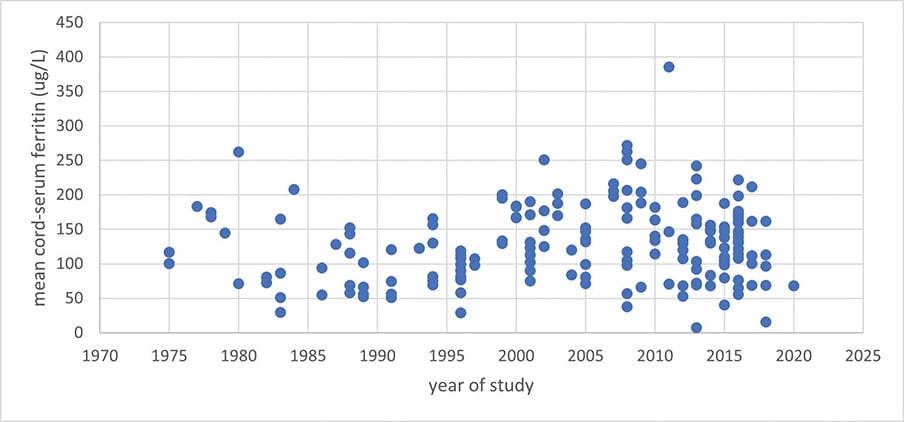
‘Normal serum-ferritin during pregnancy’ clearly stated that severe anaemia is uncommon in Australia, and specifically targeted the over-investigation and unnecessary treatment of non-pathological pregnancies with IV iron, not appropriate treatment of severe anaemia. The claim that ‘neonates born to iron deficient mothers can develop iron deficiency with or without anaemia’ refers to a cohort study of high-risk American adolescents prone to IDA prior to pregnancy whose babies had cord haemoglobin <130g/L in 24% of cases.23 The majority of literature is in overwhelming agreement that neonates are not born anaemic unless maternal anaemia is severe (<90g/L).24 25 26 27 28 29 30 31 32 33 McCarthy et al34 found cord-ferritin assays <76ug/L in 8% of neonates but did not find any association between this and any cognitive, neurological or behavioural outcomes in their whole-data analysis. The only positive findings were an increase in childhood behavioural difficulties amongst children born via caesarean section with low cord-ferritin (positive at two and five years of age), and in those born with low cord-ferritin and to obese mothers at five years of age, but not earlier. The authors stressed caution regarding low numbers and extensive adjustments to their modelling, and the paper made no comment on maternal anaemia, iron parameters or iron therapy. Cord-ferritin as a gauge of fetal iron deficiency is inconsistent (Figure 1), and unless maternal anaemia is severe, most studies have found it is independent of maternal haemoglobin, maternal ferritin, fetal haemoglobin, or iron therapy.35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52
Figure 1. Mean cord-ferritin assays from >100 studies reported in the literature between 1975–2020 (adapted from data presented in Delaney et al and Zhang et al)21,22
‘…the breadth of evidence supporting the negative effects of obstetric iron deficiency’, cites a small study in support of improved quality of life (QOL) measures amongst anaemic women after randomisation to iron therapy,53 yet these women were not pregnant. This oversight is made worse by ignoring placebo-controlled RCT data that shows iron therapy does not improve fatigue or general wellbeing in non-anaemic iron bereft subjects.54 Further, a study that focused on QOL outcomes specific to pregnancy comparing IV versus oral iron found that many positive QOL indicators, whilst initially improved with IV iron, failed to persist at delivery or postpartum, despite patients being loaded with supraphysiological doses of iron.55 Placebo aside, an improvement in physical QOL indicators immediately after IV iron may be attributed to an immediate inflammatory reaction rather than iron providing cellular energy. IV iron also has the propensity to decrease energy due to well-known side effect of hypophosphataemia – phosphate is a key component of the Krebs cycle.
Logically, if haemoglobin is used as a trigger to transfuse blood, then iron therapy should decrease the number of transfusions in the event of haemorrhage, and proportionally more benefits should be realised in populations with a higher incidence of anaemia, but this has not been realised in practice.56 57 58 Dr Clarke et al have ignored these landmark trials, and instead appealed to a multi-interventional study which reported a decrease in the number of postpartum transfusions administered to women with Hb 70–100g/L, but not <70g/L, having instigated the following interventions:
- increased education regarding the clinical threshold to transfuse;
- encouraging single unit blood transfusions, and
- increased antenatal screening with serum-ferritin and use of oral iron.59
It is not possible to determine how much of the reported decrease in blood transfusions may have been due to the latter. Screening for iron deficiency with a serum ferritin cut off of 30ug/L renders an inordinate number of pregnant women pathological, and in settings with a low prevalence of anaemia and a low postpartum haemorrhage rate, the number needed to treat is exorbitant.
Failing to treat ‘iron deficiency’ during pregnancy is not ‘analogous to tolerating maternal hypothyroidism.’ Failing to treat overt hypothyroidism is negligent. Treating hypoferremia in low-risk pregnant women with a healthy haemoglobin is of no proven benefit. Svanberg’s ingenuous iron isotope experiments demonstrated that first trimester iron absorption is actively reduced compared to the non-pregnant state.60 Early pregnancy prophylactic oral iron is often counterproductive because of this – unpleasant side-effects are largely due to poor absorption and excess gut iron. Women who are unnecessarily trialled on oral iron in early pregnancy often ‘fail’ therapy due to poor tolerance which creates an aversion to oral iron and predisposes women to avoidance of it later in pregnancy when it is readily absorbed and more likely to be of benefit if needed.
Both intolerance to oral iron and refractory serum-ferritin <30ug/L are common pregnancy scenarios, and many obstetricians revert to IV iron in these scenarios regardless of anaemia.61 It is irrefutable that routine screening for iron deficiency and/or routine oral iron therapy has contributed to the increased use of IV iron during pregnancy. Whether this consequence is intended or not is irrelevant, and it is naïve to claim early prophylactic oral iron and/or more ferritin screening will reduce this trend. Pharmaceutical companies profit handsomely from routine treatment and universal screening, and have manipulated modern antenatal care into an iron protocol to take advantage of it. This contrasts with the targeted approach to high-risk antenatal anaemia currently recommended by RANZCOG – screening with a full blood count and judicious use of oral iron, which is logical, safe, and cost-effective. ‘Normal serum ferritin in pregnancy’ did not state that doctors should wait until patients ask for iron but expressed appropriate caution that IV iron should not be administered purely on maternal request, or for unsubstantiated reasons. The term ‘hypoferritinaemia’ does not exist in the article – the accusation it was used as an incorrect label is absurd.
Dr Barton L Smith, BSc (hons), PhD, MBBS, FRANZCOG
References
- Cronin SJF, Woolf CJ, Weiss G, et al. The Role of Iron Regulation in Immunometabolism and Immune-Related Disease. Front Mol Biosci. 2019;6:116.
- Musallam KM, Taher AT. Iron deficiency beyond erythropoiesis: should we be concerned? Curr Med Res Opin. 2018;34(1):81-93.
- Murray-Kolb LE, Beard JL. Iron treatment normalizes cognitive functioning in young women. American Journal of Clinical Nutrition. 2007(85):778-87.
- Benson C, Shah A, Frise M, et al. Iron deficiency anaemia in pregnancy: A contemporary review. Obstetric Medicine. 2020.
- Murray-Kolb LE, Beard JL. Iron treatment normalizes cognitive functioning in young women. American Journal of Clinical Nutrition. 2007(85):778-87.
- Perez EM, Hendricks MK, Beard JL, et al. Mother-infant interactions and infant development are altered by maternal iron deficiency anemia. J Nutr. 2005;135(4):850-5.
- Xu F, Roberts L, Binns C, et al. Anaemia and depression before and after birth: a cohort study based on linked population data. BMC Psychiatry. 2018;18(1):224.
- Flores CJ, Sethna F, Stephens B, et al. Improving patient blood management in obstetrics: snapshots of a practice improvement partnership. BMJ Qual Improv Rep. 2017;6(1):e000009.
- Hendrickson JE, Tormey CA. Understanding red blood cell alloimmunization triggers. American Society of Hematology Educucation Program. 2016;1:446-51.
- Lee S, Guillet R, Cooper EM, et al. Prevalence of anemia and associations between neonatal iron status, hepcidin, and maternal iron status among neonates born to pregnant adolescents. Pediatr Res. 2016;79(1-1):42-8.
- Lozoff B, Jimenez E, Hagen J, et al. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105(4):E51.
- McCarthy EK, Murray DM, Hourihane JOB, et al. Behavioral consequences at 5 y of neonatal iron deficiency in a low-risk maternal-infant cohort. American Journal of Clinical Nutrition. 2021;113(4):1032-41.
- Shah AA, Donovan K, Seeley C, et al. Risk of Infection Associated With Administration of Intravenous Iron: A Systematic Review and Meta-analysis. JAMA Netw Open. 2021;4(11):e2133935.
- Canning M, Grannell L. A stain on iron therapy. Aust Prescr. 2020;43:160–163.
- Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res. 2013;28:1793–1803.
- Rosano G, Schiefke I, Göhring UM, et al. A pooled analysis of serum phosphate measurements and potential hypophosphataemia events in 45 interventional trials with ferric carboxymaltose. J Clin Med. 2020;9:3587.
- Zoller H, Schaefer B, Glodny B. Iron-induced hypophosphatemia: an emerging complication. Curr Opin Nephrol Hypertens. 2017;26:266–275.
- Shand AW, Bell J, Henry A, et al. Rapid increase in intravenous iron therapy for women of reproductive age in Australia [published online ahead of print, 2020 May 26]. Med J Aust. 2020;10.5694/mja2.50618. doi:10.5694/mja2.50618
- Richards T, Breymann C, Brookes MJ, Lindgren S, Macdougall IC, McMahon LP, Munro MG, Nemeth E, Rosano GMC, Schiefke I, Weiss G. Questions and answers on iron deficiency treatment selection and the use of intravenous iron in routine clinical practice. Ann Med. 2021 Dec;53(1):274-285.
- Richards T, Breymann C, Brookes MJ, Lindgren S, Macdougall IC, McMahon LP, Munro MG, Nemeth E, Rosano GMC, Schiefke I, Weiss G. Questions and answers on iron deficiency treatment selection and the use of intravenous iron in routine clinical practice. Ann Med. 2021 Dec;53(1):274-285.
- Mishra A, Dave N, Viradiya K. Fatal anaphylactic reaction to iron sucrose in pregnancy. Indian J Pharmacol. 2013;45(1):93-94.
- Mishra A, Dave N, Viradiya K. Fatal anaphylactic reaction to iron sucrose in pregnancy. Indian J Pharmacol. 2013;45(1):93-94.
- Lee S, Guillet R, Cooper EM et al. Prevalence of anemia and associations between neonatal iron status, hepcidin, and maternal iron status among neonates born to pregnant adolescents. Pediatr Res. 2016;79(1-1):42-8.
- Preziosi P, Prual A, Galan P, et al. Effect of iron supplementation on the iron status of pregnant women: consequences for newborns. Am J Clin Nutr. 1997;66:1178–1182
- Agrawal RMD, Tripathi AM, Agrawal KN. Cord blood haemoglobin, iron and ferritin status in maternal anaemia. Acta Paediatr Scand 1983;72:545–8.
- Hokama T, Takenaka S, Hirayama K, et al. Iron status of newborns born to iron deficient anaemic mothers. J Trop Pediatr 1996;42:75–7.
- Lao TT, Loong EPL, Chin RKH, et al. Relationship between newborn and maternal iron status and haematological indices. Biol Neonate 1991;60:303–7.
- Barton DPJ, Joy M-T, Lappin TRJ, et al. Maternal erythropoietin in singleton pregnancies: a randomized trial on the effect of oral hematinic supplementation. Am J Obstet Gynecol 1994;170:896–901.
- De Benaze C, Galan P, Wainer R, Hercberg S. Prevention de l’anemie ferriprive au cours de la grossesse par une supplémentation martiale précoce: un essai controlé. (Prevention of iron deficiency anemia in pregnancy by using early iron supplementation: a controlled trial.) Rev Epidémiol Santé Publique 1989;37:109–18 (in French).
- Milman N, Agger AO, Nielsen OJ. Iron status markers and serum erythropoietin in 120 mothers and newborn infants. Acta Obstet Gynecol Scand 1994;73:200–4.
- Kumar A, Rai AK, Basu S, et al. Cord blood and breast milk iron status in maternal anemia. Pediatrics. 2008;121(3):e673-7.
- Singla P, Tyagi M, Shankar R, et al. Fetal iron status in maternal anemia. Acta Paediatr. 1996;85:1327–1330.
- Ali R, Ismail EAR, Nada AS. Cord blood iron profile and breast milk micronutrients in maternal iron deficiency anemia. Pediatr Blood Cancer. 2012;58:233–238.
- McCarthy EK, Murray DM, Hourihane JOB, et al. Behavioural consequences at 5 y of neonatal iron deficiency in a low-risk maternal-infant cohort. American Journal of Clinical Nutrition. 2021;113(4):1032-41.
- Delaney KM, Guillet R, Fleming RE, et al. Umbilical Cord Serum Ferritin Concentration is Inversely Associated with Umbilical Cord Hemoglobin in Neonates Born to Adolescents Carrying Singletons and Women Carrying Multiples. J Nutr. 2019;149(3):406-415.
- Zhang JY, Wang J, Lu Q, et al. Iron stores at birth in a full-term normal birth weight birth cohort with a low level of inflammation. Biosci Rep. 2020;40(12):BSR20202853.
- Shao J, Lou J, Rao R, et al. Maternal serum-ferritin concentration is positively associated with newborn iron stores in women with low ferritin status in late pregnancy. J Nutr. 2012;142(11):2004-2009. doi:10.3945/jn.112.162362
- Hay G, Refsum H, Whitelaw A, Melbye EL, Haug E, Borch-Iohnsen B. Predictors of serum ferritin and serum soluble transferrin receptor in newborns and their associations with iron status during the first 2 y of life. Am J Clin Nutr. 2007 Jul;86(1):64-73.
- Bhargava M, Kumar R, Iyer PU, et al. Effect of maternal anaemia and iron depletion on foetal iron stores, birthweight and gestation. Acta Paediatr Scand. 1989;78(2):321-2.
- Mahajan S, Aalinkeel R, Shah P, Singh S, Gupta N, Kochupillai N. Nutritional anaemia dysregulates endocrine control of fetal growth. Br J Nutr. 2008 Aug;100(2):408-17. doi: 10.1017/S000711450889438X. Epub 2008 Feb 13. Erratum in: Br J Nutr. 2008 Sep;100(3):686. Gupta, N [added]. PMID: 18272016.
- Bhargava M, Iyer PU, Kumar R, Ramji S, Kapani V, Bhargava SK. Relationship of maternal serum ferritin with foetal serum ferritin, birth weight and gestation. J Trop Pediatr. 1991 Aug;37(4):149-52. doi: 10.1093/tropej/37.4.149. PMID: 1960769.
- Ru Y, Pressman EK, Guillet R, Katzman PJ, Bacak SJ, O’Brien KO.. Predictors of anemia at birth in neonates born to women carrying multiple fetuses. Pediatr Res. 2018;84(2):199–204.
- El-Farrash RA, Ismail EA, Nada AS. Cord blood iron profile and breast milk micronutrients in maternal iron deficiency anemia. Pediatr Blood Cancer. 2012 Feb;58(2):233-8. doi: 10.1002/pbc.23184. Epub 2011 May 5. PMID: 21548016.
- Basu S, Kumar N, Srivastava R, Kumar A. Effect of Severe Maternal Iron Deficiency Anemia on Neonatal Platelet Indices. Indian J Pediatr. 2015 Dec;82(12):1091-6. doi: 10.1007/s12098-015-1775-6. Epub 2015 May 19. PMID: 25980502.
- Zhao G, Xu G, Zhou M, Jiang Y, Richards B, Clark KM, Kaciroti N, Georgieff MK, Zhang Z, Tardif T, Li M, Lozoff B. Prenatal Iron Supplementation Reduces Maternal Anemia, Iron Deficiency, and Iron Deficiency Anemia in a Randomized Clinical Trial in Rural China, but Iron Deficiency Remains Widespread in Mothers and Neonates. J Nutr. 2015 Aug;145(8):1916-23. doi: 10.3945/jn.114.208678. Epub 2015 Jun 10. PMID: 26063068; PMCID: PMC4516762.
- Kilbride J, Baker TG, Parapia LA, Khoury SA, Shuqaidef SW, Jerwood D. Anaemia during pregnancy as a risk factor for iron-deficiency anaemia in infancy: a case-control study in Jordan. Int J Epidemiol. 1999 Jun;28(3):461-8. doi: 10.1093/ije/28.3.461. PMID: 10405849.
- Zavaleta N, Caulfield LE, Garcia T. Changes in iron status during pregnancy in peruvian women receiving prenatal iron and folic acid supplements with or without zinc. Am J Clin Nutr. 2000 Apr;71(4):956-61. doi: 10.1093/ajcn/71.4.956. PMID: 10731503.
- O’Brien KO, Zavaleta N, Abrams SA, Caulfield LE. Maternal iron status influences iron transfer to the fetus during the third trimester of pregnancy. Am J Clin Nutr. 2003 Apr;77(4):924-30. doi: 10.1093/ajcn/77.4.924. PMID: 12663293.
- Rehu M, Punnonen K, Ostland V, Heinonen S, Westerman M, Pulkki K, Sankilampi U. Maternal serum hepcidin is low at term and independent of cord blood iron status. Eur J Haematol. 2010 Oct;85(4):345-52. doi: 10.1111/j.1600-0609.2010.01479.x. Epub 2010 Jul 22. PMID: 20528904.
- Stoffel NU, Zimmermann MB, Cepeda-Lopez AC, Cervantes-Gracia K, Llanas-Cornejo D, Zeder C, Tuntipopipat S, Moungmaithong S, Densupsoontorn N, Quack-Loetscher K et al. Maternal iron kinetics and maternal-1 fetal iron transfer in normal weight and overweight pregnancy. Am J Clin Nutr. doi:10.1093/ajcn/nqab406.
- Delaney KM, Cao C, Guillet R, Pressman EK, O’Brien KO. Fetal iron uptake from recent maternal diet and the maternal RBC iron pool. Am J Clin Nutr. 2022 Apr 1;115(4):1069-1079. doi: 10.1093/ajcn/nqac020. PMID: 35102365; PMCID: PMC8971007.
- Gupta A, Manaktala U, Rathore AM. A randomised controlled trial to compare intravenous iron sucrose and oral iron in treatment of iron deficiency anemia in pregnancy. Indian J Hematol Blood Transfus. 2014 Jun;30(2):120-5. doi: 10.1007/s12288-012-0224-1. Epub 2013 Jan 10. PMID: 24839366; PMCID: PMC4022911.
- Murray-Kolb LE, Beard JL. Iron treatment normalizes cognitive functioning in young women. American Journal of Clinical Nutrition. 2007(85):778-87.
- Keller, P., von Känel, R., Hincapié, C.A. et al. The effects of intravenous iron supplementation on fatigue and general health in non-anemic blood donors with iron deficiency: a randomized placebo-controlled superiority trial. Sci Rep 10, 14219 (2020). https://doi.org/10.1038/s41598-020-71048-0
- Khalafallah AA, Dennis AE, Ogden K, et al. Three-year follow-up of a randomised clinical trial of intravenous versus oral iron for anemia in pregnancy. BMJ Open. 2012;2(5):e000998. Published 2012 Oct 18. doi:10.1136/bmjopen-2012-000998
- Qassim A, Mol BW, Grivell RM, Grzeskowiak LE. Safety and efficacy of intravenous iron polymaltose, iron sucrose and ferric carboxymaltose in pregnancy: A systematic review. Aust N Z J Obstet Gynaecol. 2018 Feb;58(1):22-39. doi: 10.1111/ajo.12695. Epub 2017 Sep 18. PMID: 28921558.
- Neogi SB, Devasenapathy N, Singh R, Bhushan H, Shah D, Divakar H, Zodpey S, Malik S, Nanda S, Mittal P, Batra A, Chauhan MB, Yadav S, Dongre H, Saluja S, Malhotra V, Gupta A, Sangwan R, Radhika AG, Singh A, Bhaskaran S, Kotru M, Sikka M, Agarwal S, Francis P, Mwinga K, Baswal D. Safety and effectiveness of intravenous iron sucrose versus standard oral iron therapy in pregnant women with moderate-to-severe anaemia in India: a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Glob Health. 2019 Dec;7(12):e1706-e1716. doi: 10.1016/S2214-109X(19)30427-9. Erratum in: Lancet Glob Health. 2020 Dec;8(12):e1472. PMID: 31708151.
- Richards T, Baikady RR, Clevenger B, Butcher A, Abeysiri S, Chau M, Macdougall IC, Murphy G, Swinson R, Collier T, Van Dyck L, Browne J, Bradbury A, Dodd M, Evans R, Brealey D, Anker SD, Klein A. Preoperative intravenous iron to treat
- Flores CJ, Sethna F, Stephens B, Saxon B, Hong FS, Roberts T, Spigiel T, Burgess M, Connors B, Crispin P. Improving patient blood management in obstetrics: snapshots of a practice improvement partnership. BMJ Qual Improv Rep. 2017 Jun 23;6(1):e000009. doi: 10.1136/bmjquality-2017-000009. PMID: 28824807; PMCID: PMC5492477.
- Svanberg B. Iron absorption in early pregnancy – a study of the absorption of non-haeme iron and ferrous iron in early pregnancy. Acta Obstet Gynecol Scand Suppl. 1975;48:69-85. doi:10.3109/00016347509156331
- Smith-Wade S, Kidson-Gerber G, Shand A, Grzeskowiak L, Henry A. The use of intravenous iron in pregnancy: for whom and when? A survey of Australian and New Zealand obstetricians. BMC Pregnancy Childbirth. 2020 Nov 4;20(1):665. doi: 10.1186/s12884-020-03363-3. PMID: 33148203; PMCID: PMC7640437.





Leave a Reply