Understanding the pathological basis for ovarian tumour classification is important for providing informed and comprehensive treatment and management of ovarian disease. The World Health Organization ‘blue book’ Classification of Tumours of the Female Reproductive Organs is the gold-standard classification system, which underpins the diagnosis of these tumours worldwide.1 Ovarian tumours can be conceptualised in three broad areas: non-neoplastic tumours, primary ovarian neoplasms and metastatic neoplasia to the ovary. This article aims to provide a brief overview of ovarian tumours and their classification, concentrating on the more common tumours while highlighting some of the most recent changes and developments.
Non-neoplastic tumours
Benign ovarian tumours or tumour-like lesions can cause ovarian enlargement, potentially mimicking a malignancy and causing diagnostic problems, especially in younger patients of reproductive age.2 Common benign mass forming ovarian lesions include benign follicle cyst, endometriotic cyst (also known as endometrioma), corpus luteum cyst and tubo-ovarian abscess. Ovarian endometriosis is a common pathological specimen, which is usually a simple histological diagnosis requiring the confirmation of at least two of the following four histological features: endometrial stroma, endometrial epithelium, new haemorrhage or old haemorrhage (hemosiderin).3 The risk of malignant transformation is very rare in endometriosis, occurring in only 1% of cases;4 however, the absolute risk of developing epithelial ovarian cancer increases two- to three-fold in women with endometriosis.5
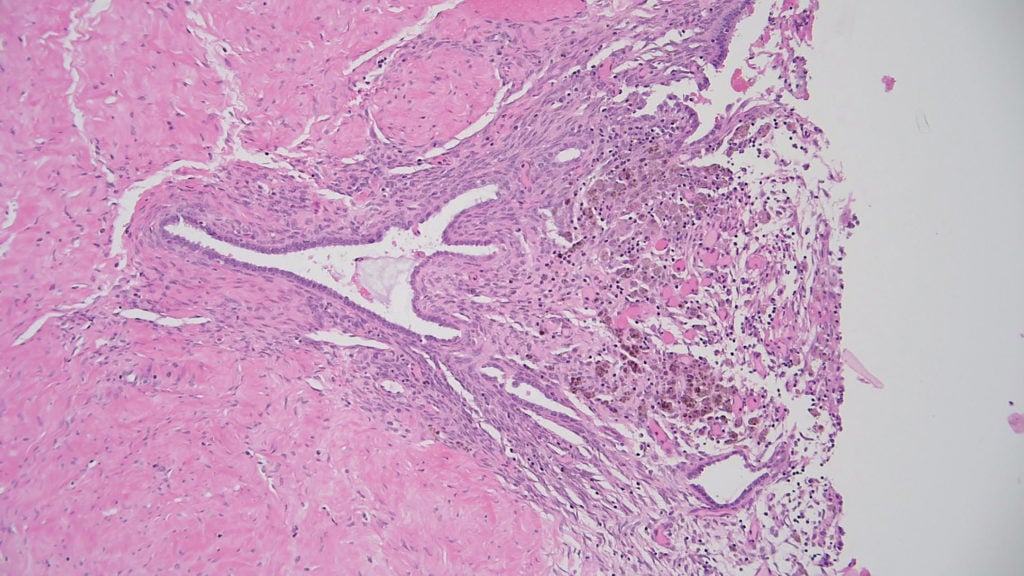
Figure 1. Endometriosis showing endometrial stroma, endometrial glands and haemosiderin-laden macrophages, confirming evidence of old haemorrhage.
Primary ovarian neoplasms
Primary ovarian neoplasms can be subdivided into three major categories: epithelial, germ cell and sex cord-stromal tumours. Other tumours, such as mesenchymal tumours, mixed epithelial and mesenchymal tumours, mesothelial tumours, lymphoid tumours and other rare and miscellaneous tumours, may also occur in the ovary.
Epithelial neoplasms
Epithelial tumours make up the bulk of the primary ovarian neoplasms, and are classified into benign, borderline (variably termed low malignant potential [LMP]) or malignant. The nomenclature for epithelial tumours is based on their cellular origin. They include, in order of most to least prevalent: serous, mucinous, endometrioid, clear cell, and Brenner tumours; mixed Müllerian tumours may occur. Seromucinous tumours are a separate tumour type included in the 2014 WHO classification;6 however, these will now be mentioned as a subtype of endometrioid tumours in the new, fifth edition of the WHO classification, due for release later in 2020.
Benign tumours are lined by simple non-stratified cells consistent with their cell of origin. Borderline tumours show increased cytological and architectural atypia compared to benign tumours, but do not demonstrate invasion. Malignant tumours show invasion of the ovarian stroma, with variable degrees of cytological atypia.
A key development in the classification of serous tumours has been the clear separation of low-grade serous carcinomas (10%) and high-grade serous carcinomas (90%).7 These two tumours do not actually represent a morphological spectrum, but rather that they are mostly different types of tumours and develop via different pathways. Low-grade serous carcinomas may contain KRAS and BRAF mutations, which may occasionally be actionable with targeted therapy. High-grade serous carcinomas are distinguished by a high level of genetic instability and harbour TP53 mutations8 in the vast majority of cases. This can be highlighted with the immunohistochemical stain p53, which shows either strong nuclear staining or complete absence of staining.9 Generally, however, low- and high-grade serous carcinomas can be reliably separated based on routine light microscopy.10 They may be associated with BRCA mutations and genetic testing is recommended for all high-grade serous cancer patients. A combination of low- and high-grade serous adenocarcinoma in the same tumour is very rare.
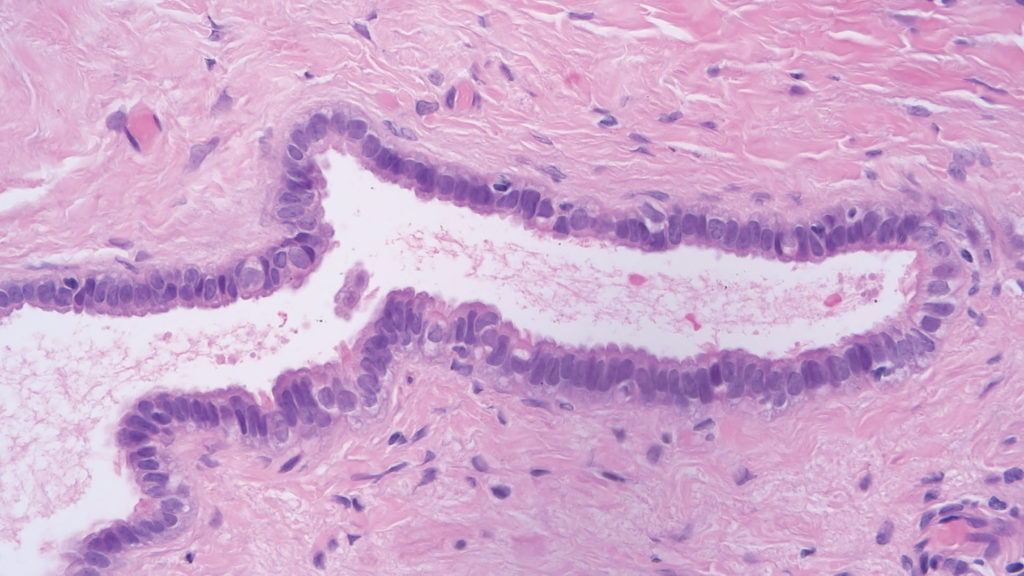
Figure 2. Benign serous cystadenoma characterised by cysts lined by benign cuboidal to columnar cells with focal ciliated cells. High power image.
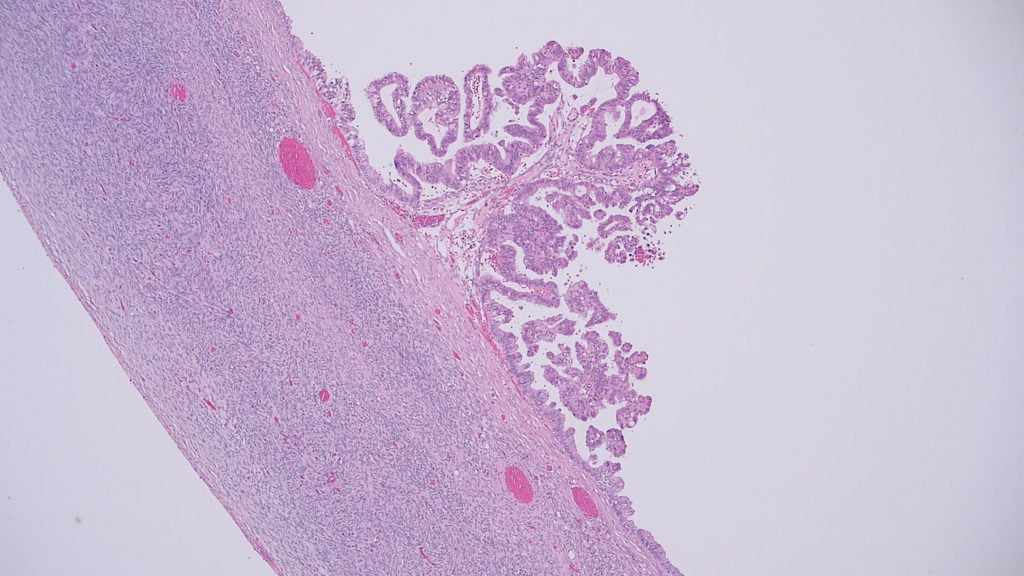
Figure 3. Serous borderline tumour showing focal hierarchical branching papillae covered by stratified serous cells showing moderately enlarged and hyperchromatic nuclei. There is no invasion present.
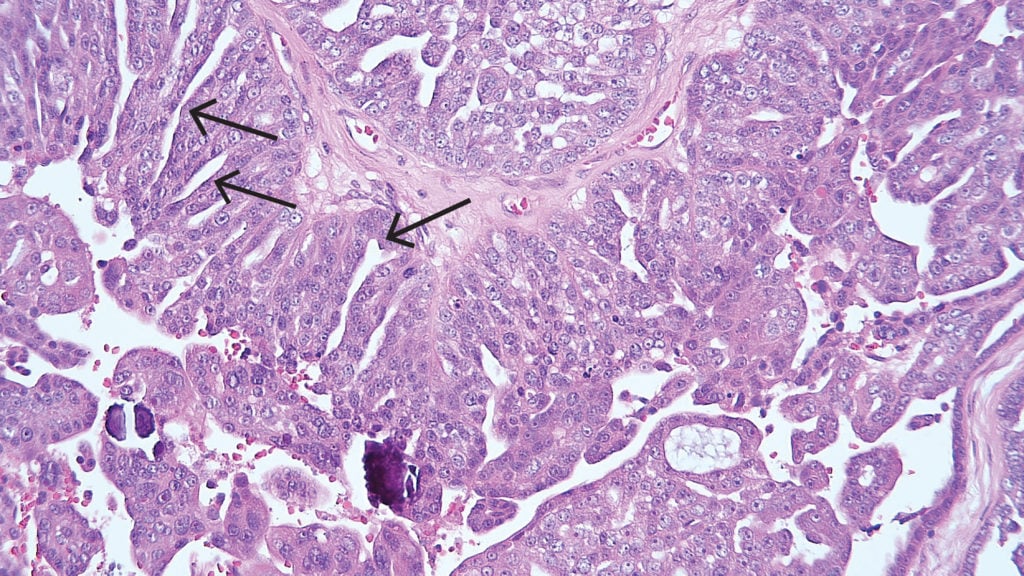
Figure 4. High-grade serous carcinoma demonstrating sheets of cells with slit-like spaces (arrows). The tumour cell nuclei are pleomorphic with prominent nucleoli. Psammoma bodies are also present.
Ovarian germ cell tumours
The classification system of ovarian germ cell tumours includes dysgerminoma, yolk sac tumour, embryonal carcinoma, choriocarcinoma, teratoma (immature and mature) and mixed germ cell tumour. Mature teratomas, also referred to as a ‘dermoid cyst’ or ‘mature cystic teratoma’, are the most common ovarian germ cell tumour. These tumours are benign, unless somatic-type malignant transformation occurs, such as squamous cell carcinoma in skin components, which is extremely rare.11 Unlike mature teratomas, immature teratomas have malignant potential and tend to be more solid than mature teratomas.12 Grading is based on the amount of immature neuroepithelial tissue present microscopically.13 Macroscopically, neural tissue typically appears soft and fleshy showing a yellowish to grey colour.14
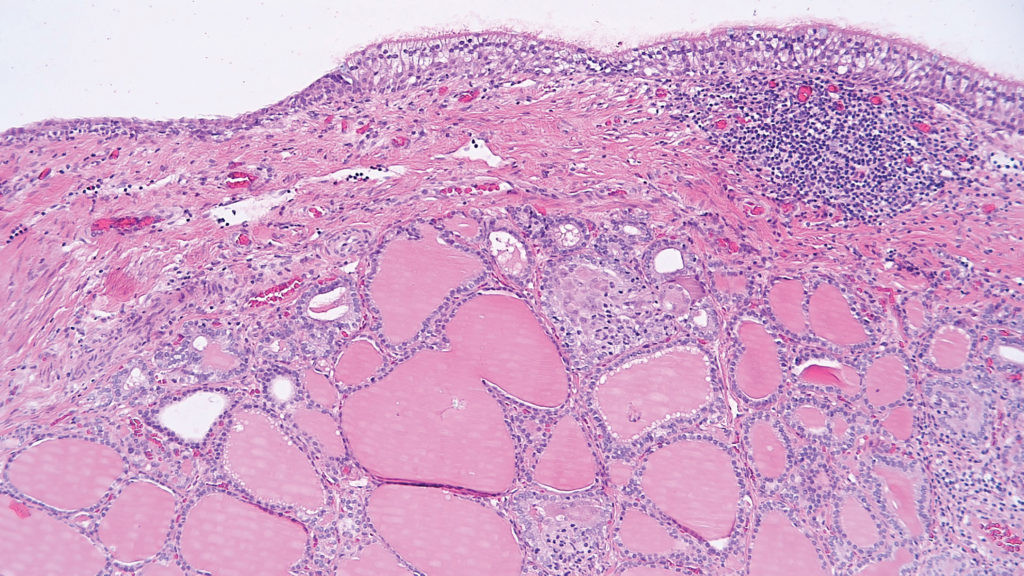
Figure 5. Mature teratoma showing a cyst lined by respiratory type epithelium with underlying thyroid tissue; often termed struma if constituting more than 50% of the lesion.
Sex cord-stromal tumours
The classification of sex cord-stromal tumours can be divided into pure stromal tumour, pure sex cord tumour or mixed sex cord-stromal tumour. Included within pure stromal tumours are benign fibroma, Leydig cell tumour, microcystic stromal tumour and other rare tumours. Pure sex cord tumours include adult granulosa cell tumour (AGCT), juvenile granulosa cell tumour (JGCT), Sertoli cell tumour and sex cord tumour with annular tubules. Tumours composed of variable proportions of Sertoli cells and Leydig cells constitute mixed sex cord-stromal tumours and are graded as well, moderately or poorly differentiated. Apart from fibromas, all of these tumours are rare, and show a vast range of histological appearances. The recent discovery of FOXL2, DICER1 and CTNNB1 mutations in AGCT, Sertoli-Leydig cell tumours and microcystic stromal tumours respectively,15 can be helpful in the diagnosis of difficult cases. Determining DICER1 mutation status can also assist with the risk assessment for DICER1 syndrome in Sertoli-Leydig cell tumours.16
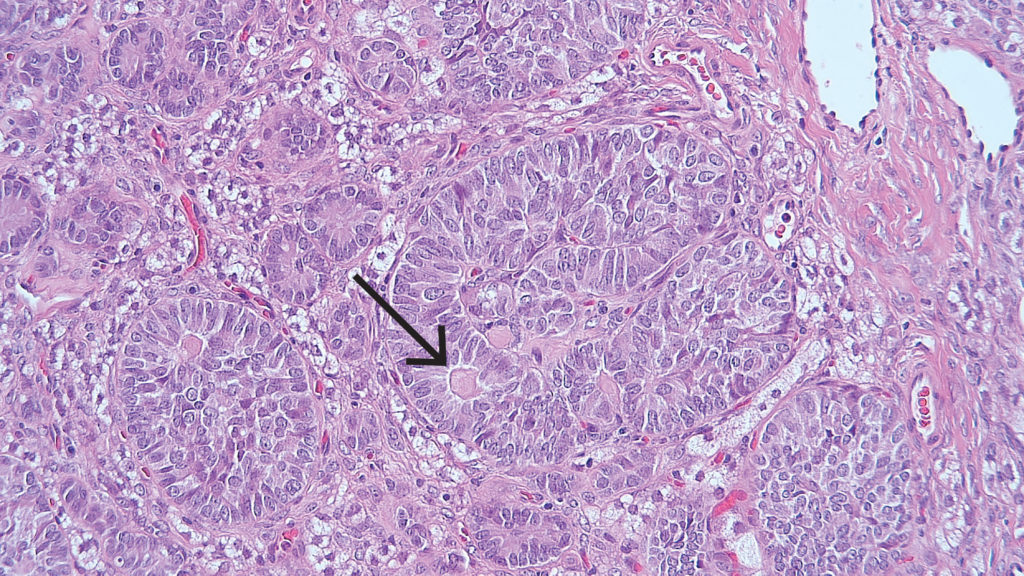
Figure 6. Adult granulosa cell tumour demonstrating a microfollicular pattern with classic Call-Exner bodies (arrow).
Metastatic tumours
It is estimated that 3–15% of ovarian malignancies are metastatic in origin and may be the presenting sign of a non-ovarian primary.17 One of the most challenging diagnostic dilemmas is differentiating primary mucinous ovarian tumour from metastatic mucinous tumour. Macroscopic features that favour metastases include small size (often less than 10 cm), bilateral tumours, surface involvement, and extensive intra-abdominal spread.18 Additional microscopic features may also raise suspicion of metastases. For instance, in a low-grade appendiceal mucinous neoplasm, the mucinous glands are scalloped and show subepithelial clefts.19 Common primary mucin producing neoplasms that metastasise to the ovaries include the gastrointestinal tract, pancreas, breast and other gynaecological sites such as cervix.
Metastasis occurring from other gynaecological sites to the ovary represents a unique and fascinating situation where the clinical behaviour in a significant amount of these cases is more aligned with that of a low-stage, organ-confined disease.20 21 In the case of low-grade endometrioid endometrial carcinoma, with secondary unilateral ovarian metastases, there is evidence that these could potentially be classified as FIGO stage IIIA endometrial carcinoma, with the possibility for more conservative management options.22 It is anticipated that the staging of these tumours will evolve as molecular testing becomes more prevalent.23
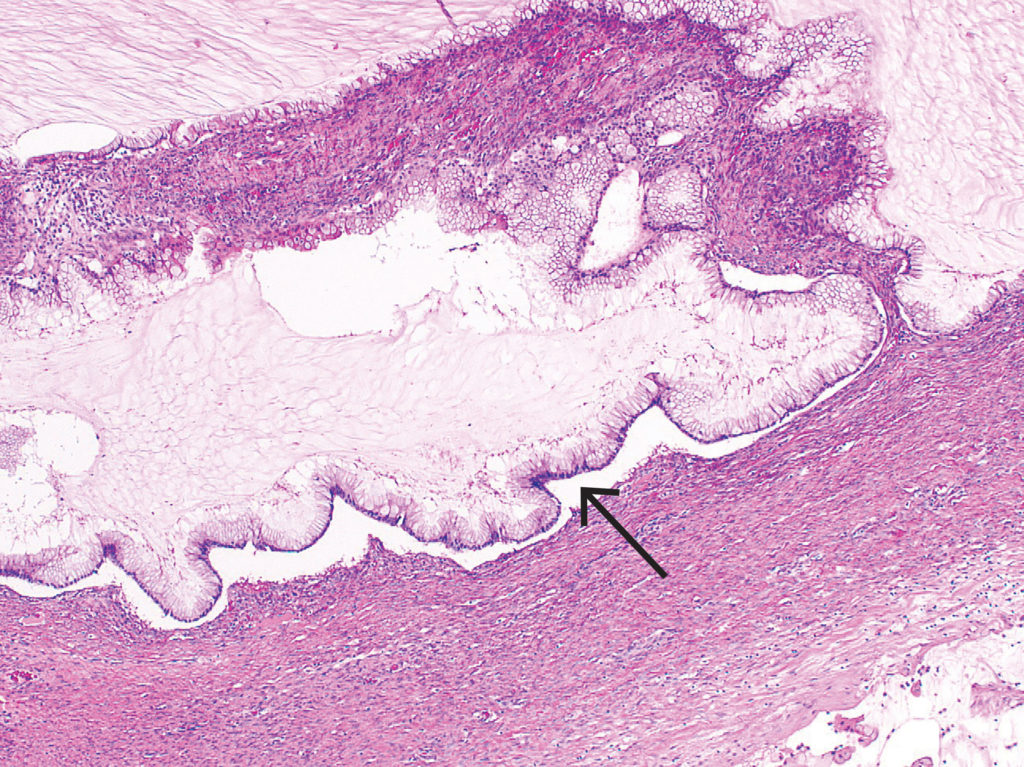
Figure 7. Metastatic low-grade appendiceal mucinous neoplasm demonstrating scalloped glands and subepithelial clefts (arrow).
Summary
The classification of ovarian tumours is extensive, with a large range of morphological appearances. These histological features, however, still form the backbone of this highly reproducible classification system24 and in the vast majority of cases, accurately predicts whether tumours will be benign, borderline or have malignant potential.
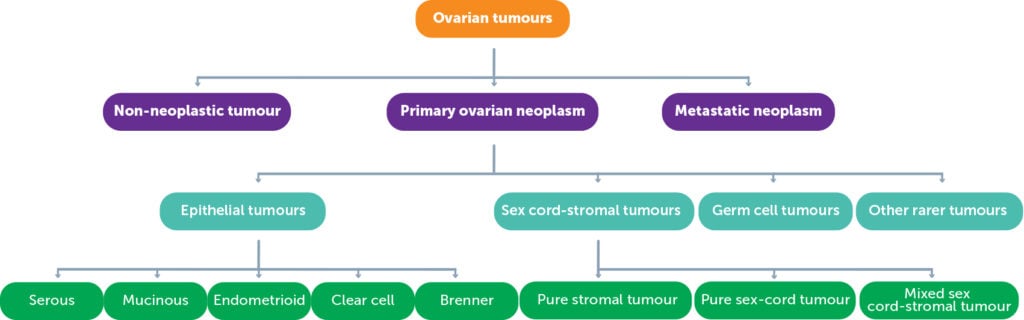
Graph 1. Summary of the classification of ovarian tumours.
I wish to thank A/Prof Lyndal Anderson for proofreading and editing this article.
References
- Kurman RJ, Carcangiu ML, Herrington CS, Young RH, (Eds.): WHO Classification of Tumours of Female Reproductive Organs. IARC: Lyons 2014.
- Crum CP, Nucci MR, Granter SR, et al. Diagnostic Gynecologic and Obstetric Pathology E-Book. Elsevier Health Sciences; 2018.
- Crum CP, Nucci MR, Granter SR, et al. Diagnostic Gynecologic and Obstetric Pathology E-Book. Elsevier Health Sciences; 2018.
- McCluggage WG. Endometriosis-related pathology: a discussion of selected uncommon benign, premalignant and malignant lesions. Histopathology. 2020;76(1):76-92.
- Wilbur MA, Shih IM, Segars JH, Fader AN. Cancer implications for patients with endometriosis. Seminars in Reproductive Medicine. 2017;35(1):110-6.
- Kurman RJ, Carcangiu ML, Herrington CS, Young RH, (Eds.): WHO Classification of Tumours of Female Reproductive Organs. IARC: Lyons 2014.
- Kurman RJ, Carcangiu ML, Herrington CS, Young RH, (Eds.): WHO Classification of Tumours of Female Reproductive Organs. IARC: Lyons 2014.
- Kurman RJ, Carcangiu ML, Herrington CS, Young RH, (Eds.): WHO Classification of Tumours of Female Reproductive Organs. IARC: Lyons 2014.
- Kurman RJ, Carcangiu ML, Herrington CS, Young RH, (Eds.): WHO Classification of Tumours of Female Reproductive Organs. IARC: Lyons 2014.
- McCluggage WG. Endometriosis-related pathology: a discussion of selected uncommon benign, premalignant and malignant lesions. Histopathology. 2020;76(1):76-92.
- Berney DM, Stoneham S, Arora R, et al. Ovarian germ cell tumour classification: views from the testis. Histopathology. 2020;76(1):25-36.
- Crum CP, Nucci MR, Granter SR, et al. Diagnostic Gynecologic and Obstetric Pathology E-Book. Elsevier Health Sciences; 2018.
- Berney DM, Stoneham S, Arora R, et al. Ovarian germ cell tumour classification: views from the testis. Histopathology. 2020;76(1):25-36.
- Crum CP, Nucci MR, Granter SR, et al. Diagnostic Gynecologic and Obstetric Pathology E-Book. Elsevier Health Sciences; 2018.
- Rabban JT, Karnezis AN, Devine WP. Practical roles for molecular diagnostic testing in ovarian adult granulosa cell tumour, Sertoli–Leydig cell tumour, microcystic stromal tumour and their mimics. Histopathology. 2020;76(1):11-24.
- Rabban JT, Karnezis AN, Devine WP. Practical roles for molecular diagnostic testing in ovarian adult granulosa cell tumour, Sertoli–Leydig cell tumour, microcystic stromal tumour and their mimics. Histopathology. 2020;76(1):11-24.
- Kurman RJ, Carcangiu ML, Herrington CS, Young RH, (Eds.): WHO Classification of Tumours of Female Reproductive Organs. IARC: Lyons 2014.
- Leen SL, Singh N. Pathology of primary and metastatic mucinous ovarian neoplasms. Journal of Clinical Pathology. 2012;65(7):591-5.
- Stewart CJ, Ardakani NM, Doherty DA, Young RH. An evaluation of the morphologic features of low-grade mucinous neoplasms of the appendix metastatic in the ovary, and comparison with primary ovarian mucinous tumours. Int J Gynecol Pathol. 2014;33(1):1-10.
- Casey L, Singh N. Metastases to the ovary arising from endometrial, cervical and fallopian tube cancer: recent advances. Histopathology. 2020;76(1):37-51.
- Singh N, Gilks CB. The changing landscape of gynaecological pathology: WHO 2020 and beyond. Histopathology. 2020;76(1):2-5.
- Casey L, Singh N. Metastases to the ovary arising from endometrial, cervical and fallopian tube cancer: recent advances. Histopathology. 2020;76(1):37-51.
- Stewart CJ, Ardakani NM, Doherty DA, Young RH. An evaluation of the morphologic features of low-grade mucinous neoplasms of the appendix metastatic in the ovary, and comparison with primary ovarian mucinous tumours. Int J Gynecol Pathol. 2014;33(1):1-10.
- Singh N, Gilks CB. The changing landscape of gynaecological pathology: WHO 2020 and beyond. Histopathology. 2020;76(1):2-5.





Leave a Reply