A 36-year-old, woman, who had been pregnant 14 times and delivered two babies at full term (G14P2), presented to our Early Pregnancy Assessment Service (EPAS). Her obstetric history included two full-term pregnancies, ten previous first-trimester miscarriages and two pregnancy terminations. Her previous full-term pregnancies were low-risk and delivered vaginally. She also had a stroke in 2015 and gastric sleeve surgery in 2023. In April 2024, we diagnosed her with a missed miscarriage of a twin pregnancy at nine weeks and she opted for surgical management via suction and evacuation.
Her beta-human chorionic gonadotropin (b-HCG) level prior to surgical evacuation was 168,933 IU/L, which is unusually high (likely related to a twin pregnancy). The procedure was uneventful, with histology confirming pregnancy-related tissue, known as products of conception (POC), however chromosomal microarray on the POC could not be completed due to insufficient tissue.
At fifteen weeks post miscarriage, she was referred back to our unit by her GP due to persistent irregular vaginal bleeding and cramping abdominal pain, and persistent low b-HCG levels ranging between 126-161 IU/L. A transvaginal pelvic ultrasound reported remaining POC in the upper uterine cavity, measuring 49x45x44 mm (51 mL), with prominent vascularity at the periphery of the collection.
In July 2024, a diagnostic hysteroscopy unexpectedly revealed an empty uterine cavity, with no remaining POC. The discrepancy in the findings between imaging and hysteroscopy led to a discussion at our combined gynaecology-radiology multidisciplinary team review. On re-examining the images, a large vascular lesion in the uterine muscle (myometrium) was identified—initially mistaken for retained tissue. Her persistent low positive b-HCG and ultrasound findings raised suspicion of Gestational Trophoblastic Disease/Neoplasia (GTD/GTN) a group of rare pregnancy-related tumours.
The initial tissue samples were re-examined using immunohistochemistry (a lab technique to detect specific markers). No abnormal or cancerous cells were found. Tests for human placental lactogen (a hormone produced by the placenta) were negative, while P57, a marker that indicates normal placental tissue, was positive.
A CT scan of the abdomen and pelvis was recommended to better understand the lesion and check for any other abnormalities or swollen lymph nodes. The case was then referred to the gynaecological oncology team at our tertiary hospital.
CT imaging of the abdomen and pelvis revealed a cystic (fluid-filled) lesion mainly in the uterine muscle at the top of the uterus (fundus), measuring 54×49×51mm. The lesion showed irregular blood vessel patterns and increased blood flow (figure 1), raising suspicion of an invasive mole (a type of GTD where abnormal tissue grows into the uterine muscle). There was no spread into the uterine lining (endometrial cavity) or beyond the outer surface of the uterus (serosa), and no lymph node involvement was detected. A biopsy was not recommended because the lesion had an extensive blood supply, increasing the risk of severe bleeding. Additionally, taking a biopsy could potentially make a cancerous lesion worse (a process known as upstaging). Given the persistently low β-hCG levels for over 15 weeks after the miscarriage, the team suspected a placental site trophoblastic tumour, a rare type of pregnancy-related growth. However, a definitive tissue diagnosis (histology) was necessary to confirm the lesion’s exact nature and guide the next steps in treatment.
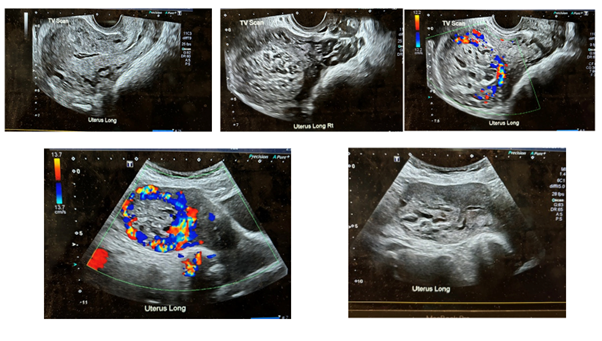
Figure 1: Ultrasound and doppler imaging of the lesion
After lengthy discussions with the patient and confirmation of no desire to preserve her future fertility, a plan was made to proceed with a hysterectomy. A total laparoscopic hysterectomy (TLH) (removal of the uterus) with bilateral salpingectomy (removal of both fallopian tubes) was performed in October 2024 by a gynaecological oncologist. During surgery, a 6 cm cystic nodule (Figure 2) was observed in the uterine fundus. The surgery and her recovery were uneventful.
Despite thorough evaluation, including additional genetic testing (cytogenetic analysis) and special tissue staining, it remained challenging to reach a definitive diagnosis even after we consulted two expert gynaecological pathologists. They concluded that the lesion represented an unusual form of an invasive mole, which likely developed from a twin pregnancy where one twin was normal, and the other was a complete hydatidiform mole (an abnormal pregnancy with no viable fetus).
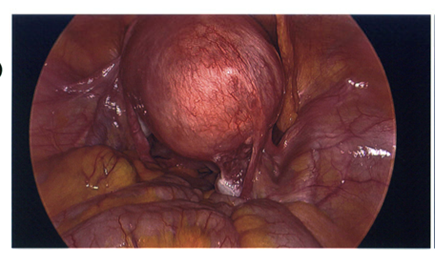
Figure 2: Laparoscopic image of the uterine fundal lesion at TLH
Discussion
Gestational trophoblastic disease (GTD) is a term that encompasses a wide range of conditions arising from abnormal development of placental tissue. These disorders include hydatidiform moles (HMs), invasive moles, gestational choriocarcinoma, and placental site trophoblastic disease 1. The main concern with GTD is a potential of development of GTN, which can be serious and potentially fatal if diagnosed at an advanced stage. Recent advances leading to earlier diagnosis and effective management have led to reduction in mortality and serious morbidity.
While high β-hCG levels are typical for invasive moles, this patient had persistently low β-hCG for over 15 weeks after miscarriage. The misinterpretation of the myometrial lesion as retained pregnancy tissue initially delayed the correct diagnosis, and the hysteroscopy provided limited insight. This case also highlights diagnostic dilemmas on histology post hysterectomy, requiring additional investigations and expert gynaecological-pathologists opinion.
While an extensive literature review is beyond the scope of this article, below are some key take home messages from this case.
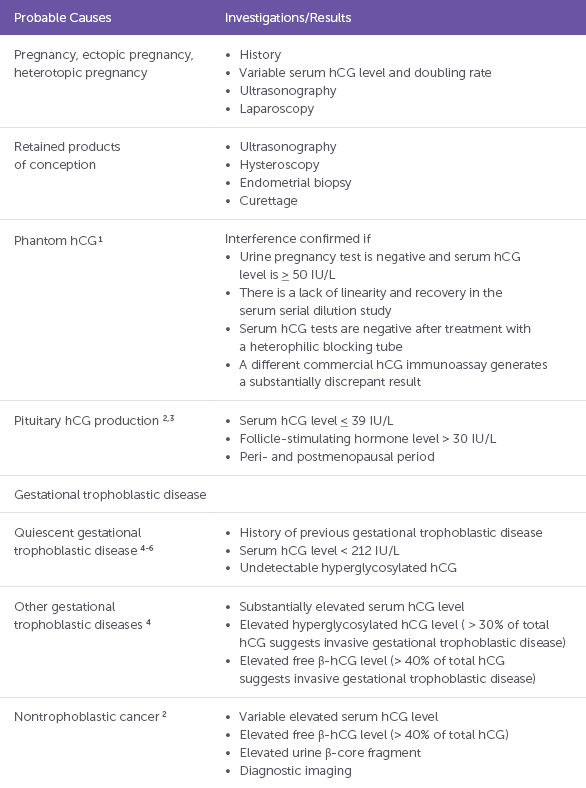
Note: hCG = human chorionic gonadotropin Table 1: Causes of persistent low β-HCG (Source: Jianing Chen et al CMAJ, December 6, 2016, 188(17–18)
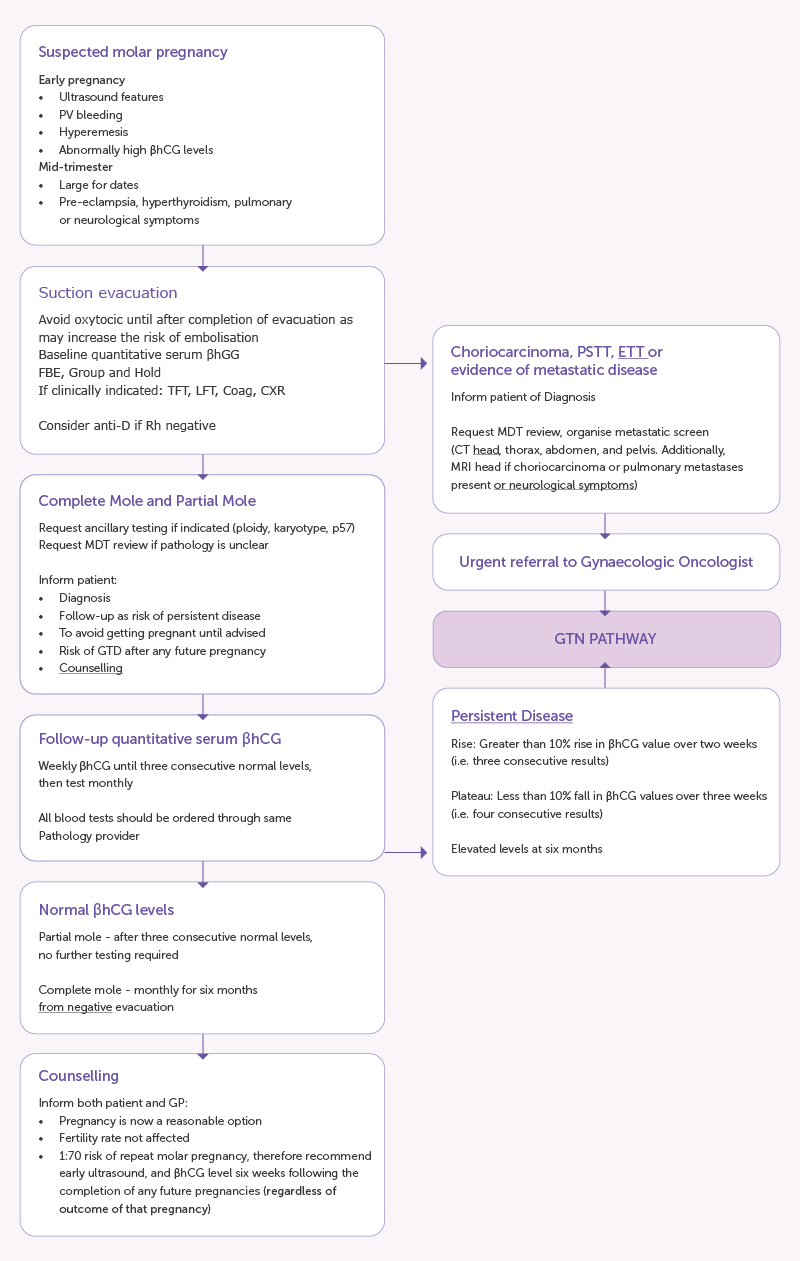
Figure 3: Management of GTD (Source- RANZCOG Statement on Management of gestational trophoblastic disease
- Persistent low b-HCG level post miscarriage deserves attention: b-HCG usually drops between 9-35 days post miscarriage. Persistent low-level elevation of β-hCG defined as less than 250 IU/L for more than three months is associated with benign and malignant conditions. Approximately 7.6% of patients with persistent low-level elevation of β-hCG have active malignancies, and it is very important to identify when low-level elevation of β-hCG is due to malignancy 2. Among GTNs, invasive moles and choriocarcinoma are usually straightforward to diagnose as they have a relatively high level of β-hCG. Placental site trophoblastic tumours (PSTT) and epithelioid trophoblastic tumours (ETT) are associated with a low level of β-hCG combined with a hypervascular or hypovascular tumour in the myometrium (like the findings in our case). Table 1 summarises the potential causes and evaluation of persistent low b-HCG 3.
- Imaging findings can be non-specific, and it may be difficult to discriminate tumours from benign conditions such as remnants of conception (as reported initially in our case) or ectopic pregnancies 4,5. It is worth mentioning that ectopic pregnancies can also occur in the myometrium (intramural pregnancy). Intramural pregnancies (IMP) are very rare and represent ≤ 1% of ectopic pregnancies (EPs). Despite a few reported cases, there is limited awareness and knowledge among sonographers and physicians 6.
- Management of patients with persistent low-level elevation of β-hCG is still controversial. Chemotherapy may be unnecessary and ineffective in the early stage of persistent low-level elevation of β-hCG because of the possibility of spontaneous cure. However, if there is radiologic evidence of a mass, treatment is recommended, though there are no specific guidelines on whether to perform surgery or chemotherapy. Generally, unless there is evidence of a definite malignancy, it can be helpful to try chemotherapy first and see if β-hCG returns to a normal level. As PSTT and ETT are chemo resistant, the response to chemotherapy is more associated with a benign condition than malignancy 7,8.
- Discrepancy between imaging findings and hysteroscopic evaluation in cases of suspected retained products of conception warrants further evaluation with serial β-hCG and further imaging.
- Management of GTD/GTN almost always requires a multidisciplinary approach including gynaecologists, gynaecological-oncologists, medical oncologists, radiologists, gynaecological-pathologists, nursing teams and support networks. Various societies have outlined guidelines for the management. Clinicians in Australia and Aotearoa New Zealand are encouraged to follow the RANZCOG guidelines. Figure 3 from the RANZCOG guideline summarises the management steps and referral pathways for patients with GTD 9.
Acknowledgements
- Dr Michael Burling – Consultant Gyne-oncologist. Liverpool Hospital, NSW, Australia
- Dr Sacha Kobilsky – Director of Radiology. Campbelltown Hospital, NSW, Australia
- Dr Adriana Suker – Registrar in Obstetrics and Gynecology. Liverpool Hospital, NSW Australia.
- Department of O&G and Early Pregnancy Assessment Service, Campbelltown Hospital NSW Australia.
Dr Sonal Karia and Dr Sharadha Mallaiyan work in the Department of O&G at Campbelltown Hospital, Sydney Southwest LHD, NSW, Australia.
References
- Monchek, Ruth, Wiedaseck, Susan. Gestational trophoblastic disease An overview. Journal of Midwifery and Women’s Health May-June 2012, Volume 57 ( 3 ), p 255 – 259
- Cole LA, Khanlian SA, Giddings A, Butler SA, Muller CY, Hammond C, et al. Gestational trophoblastic diseases: 4. Presentation with persistent low positive human chorionic gonadotropin test results. Gynecol Oncol. 2006;102:165–172.
- Jianing Chen, Sheri-Lee Samson, James Bentley, Yu Chen. Persistent mild increase of human chorionic gonadotropin levels in a 31-year-old woman after spontaneous abortion. CMAJ, December 6, 2016, 188(17–18)
- Seckl MJ, Sebire NJ, Berkowitz RS. Gestational trophoblastic disease. Lancet. 2010;376:717–729.
- Lima LL, Parente RC, Maestá I, Amim J, Junior, de Rezende Filho JF, Montenegro CA, et al. Clinical and radiological correlations in patients with gestational trophoblastic disease. Radiol Bras. 2016;49:241–250.
- Carnot N. Ntafam a,*, ItunuOluwa Sanusi-Musa b, Robert D. Harris. Intramural ectopic pregnancy: An individual patient data systematic review. Eur J Obstet Gynecol Reprod Biol X . Volume 21; March 2024, 100272
- Laurence A Cole, Sarah A Khanlian, Almareena Giddings, Stephen A Butler, Carolyn Y Muller, Charles Hammond, Ernest Kohorn. Gestational trophoblastic diseases: 4. Presentation with persistent low positive human chorionic gonadotropin test results. Gynecol Oncol 2006 Aug;102(2):165-72
- Ji-Hye Kim, Sun Kyung Lee, Soo Hyun Hwang, Jung-Sun Ki, Gun Yoon2, Yoo-Young Lee, Tae-Joong Kim,Chel Hun Choi, Byoung-Gie Kim, Duk-Soo Bae, Jeong-Won Lee. Extrauterine epithelioid trophoblastic tumor in hysterectomized woman. Obstet Gynecol Sci 2017;60(1):124-128
- https://ranzcog.edu.au/wp-content/uploads/Management-Gestational-Trophoblastic-Disease.pdf







Leave a Reply