Caesarean scar pregnancy (CSP) is a rare yet significant obstetric condition that occurs when a pregnancy implants over or within the scar from a previous caesarean section. Although its true incidence remains unclear, CSP appears to be on the rise due to higher caesarean section (CS) rates and improved early pregnancy ultrasound detection 1–3. Importantly, CSP carries elevated risks of maternal haemorrhage, preterm birth, and, in some cases, hysterectomy. This article explores the definition of CSP, highlights its risk factors, and outlines current management strategies—all crucial for informed decision-making and optimal patient outcomes.
What is CSP?
CSP occurs when a pregnancy implants over a caesarean scar or within a defect or ‘niche’ following a CS 1. Because it is an iatrogenic condition (a condition that is the result of another medical treatment), CPS incidence is increasing, largely due to increasing CS rates, enhanced early pregnancy ultrasound techniques, and growing clinician awareness 2. Despite these developments, the precise incidence remains uncertain 3.
Why is CSP important?
CSP is associated with an increased risk of significant maternal and perinatal morbidity and mortality due to maternal haemorrhage, unplanned hysterectomy and preterm birth 2.
There is a growing understanding that CSP and placenta accreta spectrum (PAS) likely lie on a continuum, given they share risk factors and histological features 4. Notably, up to 75% of expectantly managed CSPs progress to PAS 5.
What are the major risk factors for CSP?
A history of CS is the primary risk factor for CSP 6. However, the contribution of the number of prior CS remains unclear 3. Although some suggest that the technique used to close the hysterotomy could influence CSP development, no optimal method (e.g. single versus double-layer closure or whether to incorporate the decidua to prevent the development of a CS niche) has been definitively identified 3,7.
An interpregnancy interval of less than two years following CS may increase the risk of CSP 8. Current recommendations for an interpregnancy interval of at least 12 to 18 months following CS 9-11 focus mainly on the risk of scar rupture in labour. Further research is required to determine the impact of this interpregnancy interval on CSP risk.
How can CSP present clinically?
Clinical presentations of CSP vary widely, ranging from asymptomatic cases to vaginal bleeding (with or without pelvic pain), through to severe events such as a presentation with uterine rupture and haemodynamic collapse 2.
How is CSP diagnosed?
Early diagnosis allows timely counselling and management, ultimately leading to lower maternal complication rates 12,13. Transvaginal ultrasound in the first trimester of pregnancy is currently the gold standard for diagnosis of CSP 1 and ideally should be performed at 5-7 weeks of gestation 6. This timing is crucial because the ultrasound appearance changes rapidly during early pregnancy as the gestational sac expands.
Several classification systems aim to aid CSP identification and prognosis 14-16. These systems generally focus on how deeply the gestational sac implants towards an anterior uterine surface or bladder, rather than towards the endometrial cavity. The simplest classification criteria makes a distinction between ‘on the scar’ implantation (implantation of the gestational sac over a caesarean scar, with residual myometrium between the gestational sac and the anterior uterine surface or bladder) and ‘in the niche’ implantation (where the sac resides within a niche or defect with thinned myometrium 14 between the gestational sac and the anterior uterine surface or bladder) 6. The latter scenario carries higher rates of uterine rupture and development of PAS 14.
Importantly, assessing a uterine niche’s presence and size is not reliable when a possible CSP is overlying it 1. Residual myometrial thickness (RMT) at the implantation site can be particularly helpful for prognosis 1, as an RMT under 1–3 mm strongly suggests CSP 2, and an RMT below 5mm is associated with PAS 5.
Image 1 and Image 2 Images showing ‘In the niche’ and ‘on the scar’ implantation of the early gestational sac.
Image 3 – Image of a CSP at nine weeks’ gestation with expansion of the gestational sac into the corpus of the uterus, thin RMT and disordered vascularity.
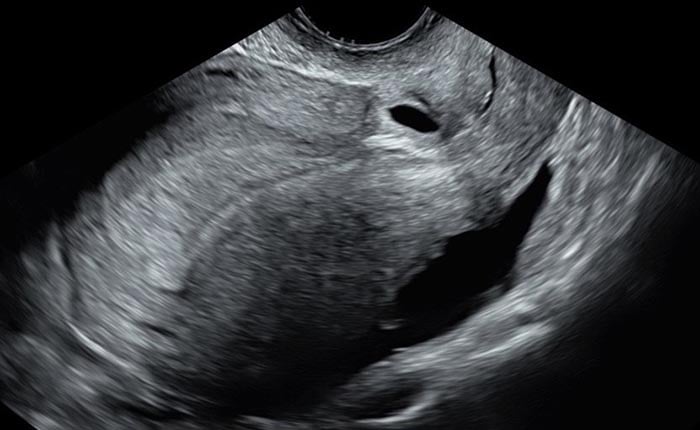
Image 1 showing ‘In the niche’ implantation of the early gestational sac
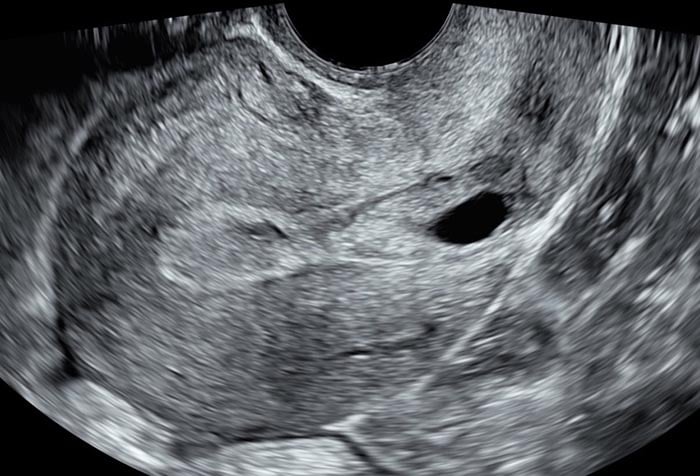
Image 2 showing ‘on the scar’ implantation of the early gestational sac
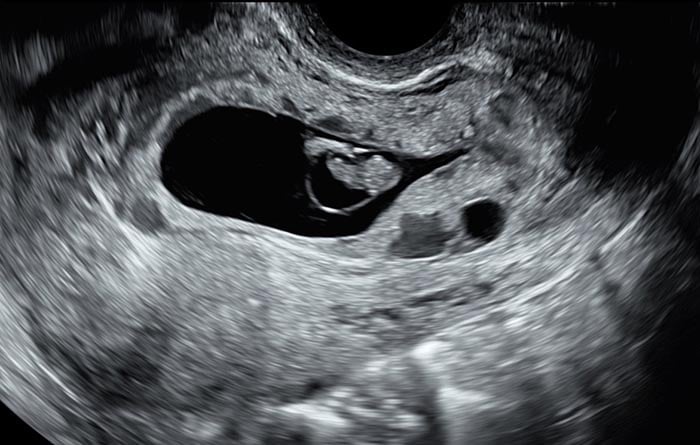
Image 3 of a CSP at nine weeks’ gestation with expansion of the gestational sac into the corpus of the uterus, thin RMT and disordered vascularity
Colour flow Doppler may help differentiate a CSP from an evolving miscarriage; however, the value of measuring Doppler flow parameters remains unclear. A miscarriage in progress may also demonstrate ‘sliding’ or movement of the gestational sac with probe pressure. MRI is unlikely to add to the diagnosis of CSP, particularly in the first trimester 1.
Notably, the usual diagnostic features of CSP are less useful following a classical caesarean section with a midline vertical scar, due the positioning of the gestational sac within the corpus of the uterus 17; this is a limitation of current diagnostic pathways.
What are the current management options?
Managing CSP is challenging, as there is no standardised management protocol. Historically, termination of pregnancy has been recommended 5. However, a more contemporary approach emphasises shared decision making, considering the individual features of CPS, likely prognosis, and desired fertility. Carefully considering these factors helps guide women to an informed choice between early termination or continuing the pregnancy.
Factors associated with a lower risk of PAS or uterine rupture include the absence of fetal heart activity; ‘on the niche’ implantation and an RMT of >5mm 5.
Despite traditional guidance favouring termination of pregnancy, expectant management can be an option in carefully selected cases. Outcomes for expectant management are dependent on the presence of fetal heart activity. In the setting of CSP without fetal heart activity in the first trimester, up to 69% of expectantly managed cases will result in uncomplicated miscarriage; however, there is still a high risk of maternal haemorrhage in 22%, and a need for further surgical or medical intervention in 31% 5. Importantly, the risk of uterine rupture in the first trimester is considered to be low 5,18.
Expectant management becomes more controversial if fetal heart activity is present, as this is associated with a higher risk of maternal morbidity 2. CSP with fetal heart activity still carries a chance of miscarriage, which may be complicated by haemorrhage or require surgical intervention. The risk of rupture in the second trimester is approximately 10%; however, this data is from registries with small case numbers. International registry data now shows that, of pregnancies that reach the third trimester, the live birth rate reaches 100%, but 74% have histological evidence of PAS, and 60% of patients require hysterectomy 5. For expectantly managed CSP with a live fetus, delivery is recommended by planned caesarean section between 34 and 36 weeks’ gestation 2, although the likelihood of hysterectomy remains high.
For those who choose to discontinue the pregnancy, earlier intervention results in less need for additional treatments 12.
Historically, gestational sac injection agents to induce embryocide (e.g., methotrexate, lignocaine, potassium chloride) were used with moderate (66%) success in resolving CSP 12.
Notably, systemic methotrexate is no longer recommended 2 as it results in low resolution rates of (59%) and higher rates of complications including methotrexate toxicity, prolonged retention of products of conception with associated delay to return to fertility 12.
Surgical intervention—whether by suction evacuation or excision—offers the highest resolution rates (over 90%) but still carries a risk of haemorrhage and hysterectomy 12. Double-balloon catheter use has shown similarly high rates of resolution when applied before eight weeks’ gestation, although close follow-up is necessary 13. In cases of severe bleeding, uterine artery embolisation is an established method for preserving the uterus 12.
What is the recurrence risk of CSP?
After CSP resolution, there is no conclusive evidence for the ideal interval before attempting another pregnancy. However, the risk of recurrence is roughly 34% 19. Consequently, women should undergo transvaginal ultrasound at 6–7 weeks’ gestation in any future pregnancy to rule out recurrent CSP.
Whether CS niche repair prevents CSP remains unclear. Some studies show increased RMT after laparoscopic or hysteroscopic niche repair 20, a lower CSP risk 21, and reduced uterine dehiscence rates 20,22,23. Nonetheless, these findings come from small cohorts with varied surgical techniques, indicating the need for further research.
Conclusion
Although CSP can be potentially daunting, early identification should be viewed as a positive step in early pregnancy care, offering a window for shared decision-making about pregnancy continuation or termination. Early pregnancy ultrasound undertaken by appropriately skilled sonographers and sonologists is crucial in the diagnosis and prognostication of CSP. The contribution of international registries and further research will further refine our understanding of CSP and its management and should allow more accurate prognostication for what is still an under-recognised condition.
Acknowledgements
My thanks go to Dr Glenn Gardener (Director of Maternal Fetal Medicine, Mater Mothers’ Hospital) for his guidance in writing this article.
References
- Jordans IPM, Verberkt C, De Leeuw RA, Bilardo CM, Van Den Bosch T, Bourne T, et al. Definition and sonographic reporting system for Cesarean scar pregnancy in early gestation: modified Delphi method. Ultrasound Obstet Gynecol. 2022 Apr;59(4):437–49.
- Society for Maternal-Fetal Medicine (SMFM). Electronic address: [email protected], Miller R, Timor-Tritsch IE, Gyamfi-Bannerman C. Society for Maternal-Fetal Medicine (SMFM) Consult Series #49: Cesarean scar pregnancy. Am J Obstet Gynecol. 2020 May;222(5):B2–14.
- Nijjar S, Jauniaux E, Jurkovic D. Definition and diagnosis of cesarean scar ectopic pregnancies. Best Pract Res Clin Obstet Gynaecol. 2023 Jul;89:102360.
- Timor-Tritsch IE, Monteagudo A, Cali G, Palacios-Jaraquemada JM, Maymon R, Arslan AA, et al. Cesarean scar pregnancy and early placenta accreta share common histology. Ultrasound Obstet Gynecol. 2014 Apr;43(4):383–95.
- Calì G, Timor-Tritsch IE, Palacios-Jaraquemada J, Monteaugudo A, Buca D, Forlani F, et al. Outcome of Cesarean scar pregnancy managed expectantly: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018 Feb;51(2):169–75.
- Timor-Tritsch IE, Monteagudo A, Calì G, D’Antonio F, Kaelin Agten A. Cesarean Scar Pregnancy: Diagnosis and Pathogenesis. Obstet Gynecol Clin North Am. 2019 Dec;46(4):797–811.
- Di Spiezio Sardo A, Saccone G, McCurdy R, Bujold E, Bifulco G, Berghella V. Risk of Cesarean scar defect following single- vs double-layer uterine closure: systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol. 2017 Nov;50(5):578–83.
- Luo L, Ruan X, Li C, Chen S, Hu Q, Mueck AO. Early clinical features and risk factors for cesarean scar pregnancy: a retrospective case-control study. Gynecol Endocrinol. 2019 Apr;35(4):337–41.
- Royal College of Obstetricians and Gynaecologists. Birth After Previous Caesarean Birth – Green-top Guideline No.45. 2015.
- Royal Australian and New Zealand College of Obstetricians and Gynaecologists. Birth after previous caesarean section – Best Practice Statement. 2019.
- The American College of Obstetricians and Gynecologists. Obstetric Care Consensus Number 8 – Interpregnancy Care [Internet]. 2021 [cited 2024 Nov 26]. Available from: https://www.acog.org/clinical/clinical-guidance/obstetric-care-consensus/articles/2019/01/interpregnancy-care
- Kaelin Agten A, Jurkovic D, Timor-Tritsch I, Jones N, Johnson S, Monteagudo A, et al. First-trimester cesarean scar pregnancy: a comparative analysis of treatment options from the international registry. American Journal of Obstetrics and Gynecology. 2024 Jun 1;230(6):669.e1-669.e19.
- Timor-Tritsch I, Buca D, Di Mascio D, Cali G, D’Amico A, Monteagudo A, et al. Outcome of cesarean scar pregnancy according to gestational age at diagnosis: A systematic review and meta-analysis. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2021 Mar 1;258:53–9.
- Agten AK, Cali G, Monteagudo A, Oviedo J, Ramos J, Timor-Tritsch I. The clinical outcome of cesarean scar pregnancies implanted “on the scar” versus “in the niche.” American Journal of Obstetrics & Gynecology. 2017 May 1;216(5):510.e1-510.e6.
- Cali G, Forlani F, Timor-Tritsch IE, Palacios-Jaraquemada J, Minneci G, D’Antonio F. Natural history of Cesarean scar pregnancy on prenatal ultrasound: the crossover sign. Ultrasound in Obstetrics & Gynecology. 2017;50(1):100–4.
- Lin SY, Hsieh CJ, Tu YA, Li YP, Lee CN, Hsu WW, et al. New ultrasound grading system for cesarean scar pregnancy and its implications for management strategies: An observational cohort study. PLoS One. 2018 Aug 9;13(8):e0202020.
- ESHRE working group on Ectopic Pregnancy, Kirk E, Ankum P, Jakab A, Le Clef N, Ludwin A, et al. Terminology for describing normally sited and ectopic pregnancies on ultrasound: ESHRE recommendations for good practice. Hum Reprod Open. 2020;2020(4):hoaa055.
- Silva B, Viana Pinto P, Costa MA. Cesarean Scar Pregnancy: A systematic review on expectant management. Eur J Obstet Gynecol Reprod Biol. 2023 Sep;288:36–43.
- Timor-Tritsch IE, Horwitz G, D’Antonio F, Monteagudo A, Bornstein E, Chervenak J, et al. Recurrent Cesarean scar pregnancy: case series and literature review. Ultrasound Obstet Gynecol. 2021 Jul;58(1):121–6.
- Jordans IPM, Vissers J, de Leeuw RA, Hehenkamp WJK, Twisk JWR, de Groot CJM, et al. Change of the residual myometrial thickness during pregnancy in women who underwent laparoscopic niche resection compared with controls without niche surgery: a prospective comparative cohort study. Am J Obstet Gynecol. 2022 Dec;227(6):901.e1-901.e12.
- Cheng XY, Cheng L, Li WJ, Qian LH, Zhang YQ. The effect of surgery on subsequent pregnancy outcomes among patients with cesarean scar diverticulum. Int J Gynaecol Obstet. 2018 May;141(2):212–6.
- Goldenberg M, Timor I, Mashiach R, Cohen S, Sasson AM. Pregnancy following cesarean scar defect (niche) repair: a cohort study. Arch Gynecol Obstet. 2022 Nov;306(5):1581–6.
- Tsuji S, Takahashi A, Higuchi A, Yamanaka A, Amano T, Kimura F, et al. Pregnancy outcomes after hysteroscopic surgery in women with cesarean scar syndrome. PLOS ONE. 2020 Dec 3;15(12):e0243421.






Leave a Reply