It is now 40 years since it was discovered that umbilical cord blood is a rich source of blood stem cells, similar to those found in bone marrow, that can produce all the mature blood cells needed in the body. Since cord blood is traditionally a waste product, discarded with the cord and placenta following delivery, it can easily be collected and stored in a cord blood bank. All the cord blood obtained from the placenta and cord of a single delivery is known as a cord blood unit. Since the first cord blood transplant in 1988, cord blood has proven to be a valuable source of stem cells for bone marrow transplant for the treatment of leukaemia and up to 80 other disease indications. There are now more than 800,000 cord blood units stored in public cord blood banks globally, with more than 40,000 cord blood transplants performed. One of the greatest advantages of using cord blood for transplant is that unlike bone marrow, the cord blood cells do not need to be a perfect tissue typing match with the patient, thereby improving the likelihood of finding a suitable donor.
The BMDI Cord Blood Bank (CBB) opened in 1996 and is located at the Royal Children’s Hospital (RCH) in Melbourne, operating as a partnership between the RCH, Murdoch Children’s Research Institute and the Fight Cancer Foundation. The bank is one of three public cord blood banks in Australia, the others being in Sydney and Brisbane. The BMDI CBB operates a Cord Blood Collection site at the Royal Women’s Hospital in Parkville, which also services Frances Perry House. Pregnant mothers can altruistically donate their cord blood to the bank, where they will be recruited by the bank’s specially trained midwives. The role of the Cord Blood Bank Collection Coordinators is to obtain consent and family and medical history, collect the cord blood and a sample of the mother’s blood for infectious disease testing, package up the cord blood for transport to the CBB Processing laboratory at the RCH and to contact the mums for long-term follow up. At the processing lab, the cord blood is tested and the white cells containing stem cells are isolated and stored in liquid nitrogen at –196°C. The cord blood donor (mother) is contacted six months later to check on her health and the health of the baby. If all is well, the information about the cord blood unit (tissue type and cell count) is uploaded to an international bone marrow donor registry where the information can be searched for patients around the world who require a stem cell transplant for the treatment of leukaemia and other disorders. The BMDI CBB has around 13,000 cord blood units stored onsite at the RCH and has released nearly 600 units for treatment of patients; approximately one-third being used in Australia and two-thirds going overseas.
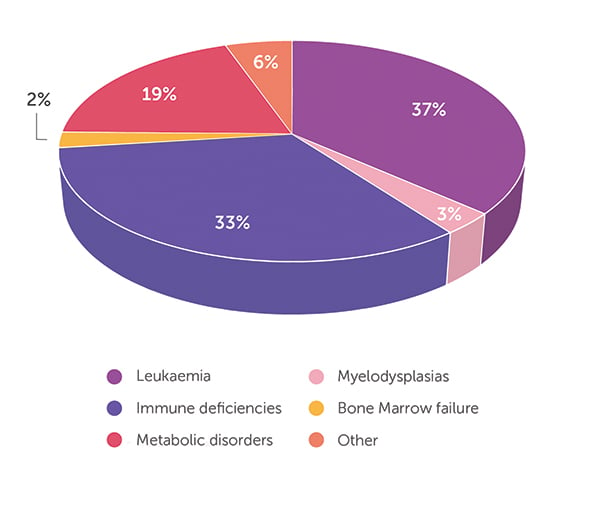
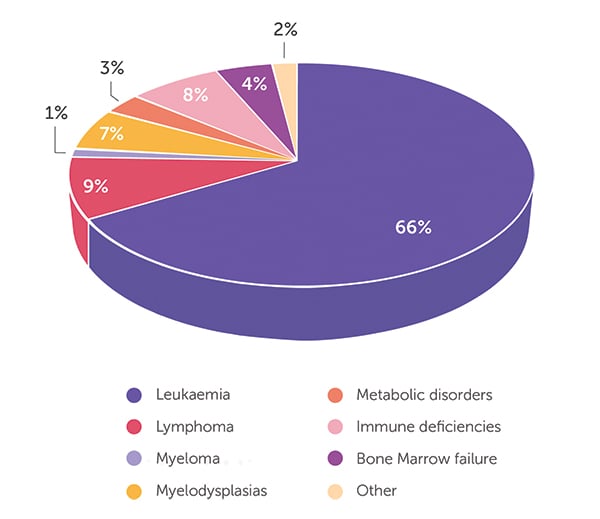
As of 30 June 2023, the BMDI CBB has released 594 cord blood units. The age of transplant recipients ranges from infants through to 76 years. When looking at all 594 releases (Figure 1), the majority (66%) of patients received cord blood for the treatment of leukaemia, followed by lymphoma (9%), immunodeficiencies (8%) and myelodysplasia (7%). Only 4% and 3% of CBU were released for the treatment of bone marrow failure and metabolic disorders, respectively, with the remaining 3% comprising a mix of different indications. A closer look at the data highlights the important role that cord blood stem cell transplant plays in the treatment of infants. Of the cord blood units released, 93 went to infants aged <2 years, with 64 cord blood units going to infants aged <1 year. The distribution of the disease indications for released cord blood is very different in infants compared with that for all ages (Figure 2). Of the 93 cord blood units shipped for treatment of infants, 37% were for metabolic disorders (eg Hurler syndrome, mucopolysaccharidosis type 1, MPS-type 1H, mucolipidosis), 33% for immune deficiencies (eg SCID-Ommen syndrome, Wiskott Aldrich, Griscelli syndrome, chronic granulomatous disease), 19% for the treatment of leukaemia (eg ALL, AML, JMML) and 5% for myelodysplasias and bone marrow failure. The 6% for ‘other’ indications includes metachromatic leukodystrophy (n = 2) and osteopetrosis (n = 3), severe disorders diagnosed in infancy. Almost half of the infants treated were Australian patients, with just over half of the cord blood units sent to treat infants overseas. With 63% of all patients treated as infants still alive, it feels appropriate that this stem cell source from newborns should play such a vital role in helping and curing other infants.
- Figure 1: Disease indication for cord blood units released for transplant (n = 594).
- Figure 2: Disease indication for cord blood units released for transplant in patients ≤2 years old (n = 93).
Looking into the future
In addition to blood stem cells, cord blood is a rich source of mesenchymal stem cells, endothelial progenitor cells, monocytes, B cells and regulatory T cells, which can function to regulate cellular signalling for growth, differentiation and immune responses via cell–cell interaction and/or paracrine mechanisms. Preclinical studies by our group and others have shown that in instances of tissue injury and inflammation cord blood is able to promote repair and new growth and dampen inflammation. Cord blood is increasingly being used to treat disorders and illnesses in infants beyond those treatable using a stem cell transplant. To date, none of these new uses are standard of care but clinical trials are being undertaken to confirm safety, feasibility and potential effectiveness.
In a ground-breaking clinical trial recently completed at the RCH, cord blood stem cell therapy has been used to treat infants born with the congenital heart abnormality hypoplastic left heart syndrome (HLHS).¹ HLHS is lethal unless the heart is re-plumbed via surgery to connect to the body’s circulation in the first few days of life. At age 3–5 months, a second-stage surgery takes place to allow a more physiological circulation. Despite decades of continuous improvements in the surgical and medical management of HLHS, morbidity and mortality remain highest between birth and the second stage of surgery (ie the interstage period). This first-in-human clinical trial has shown safety and feasibility of delivering cord blood to the heart blood vessels during first-stage surgery performed at days 2–3 of life. The trial also provides evidence that cord blood stem cell therapy might support early heart remodelling with improved function during the critical interstage period. This clinical trial used autologous (baby’s own) cord blood, but has paved the way for a planned clinical trial to assess safety and feasibility of using unrelated cord blood from the BMDI CBB in infants and children with heart abnormalities.
Cord blood stem cell therapy might also play a role in preventive or regenerative treatment of neonatal morbidities due to neurological injury. Within Australia, this field is being led by a group at Monash Children’s Hospital and the Hudson Institute of Medical Research in Clayton. Recent reviews by this group have described the status of the many early stage clinical trials in progress using cord blood for neurological and lung conditions.² A safety and feasibility study (CORD-SAFE) is currently in progress using autologous cord blood in extremely preterm infants born at less than 28 weeks, a population with a high incidence of brain injury and subsequent neurodisability.³ This autologous clinical trial is still ongoing, but the findings to date have led to the planning of a safety and feasibility study, also led by Associate Professor Atul Malhotra at Monash Children’s Hospital, using unrelated cord blood from the BMDI CBB in pre-term infants with severe brain injury (ALLO Trial). It is hypothesised that pre-term infants who demonstrate severe brain injury in the days after birth might benefit long term in their neurological function following the infusion of cord blood in the days after injury.
There have now been many clinical trials and cord blood infusions for treatment of cerebral palsy, with evidence mounting that in certain groups of patients cord blood therapy might be of benefit in improving motor outcomes. There is still much work to be done in this space. What these clinical trials have shown definitively is that both autologous and unrelated cord blood infusion is safe.⁴
Cord blood currently provides a vital source of cells for stem cell transplants for the treatment of infants with metabolic disorders, immune deficiencies and leukaemia. There are many children alive and well today because of the altruistic actions of mothers who donate their cord blood to the public cord blood banks, for potential treatment of patients they will never meet. Cord blood is set to play an even larger role in the future in the treatment of infants and others. As the many clinical trials in progress confirm safety and feasibility, the next phase of clinical trials will determine efficacy and benefit. It is truly exciting to see where the next decade leads.
References
- Brizard CP, Elwood NJ, Kowalski R, et al. Safety and feasibility of adjunct autologous cord blood stem cell therapy during the Norwood heart operation. J Thorac Cardiovasc Surg. Epub 2023 Jul 30. doi:10.1016/j.jtcvs.2023.07.035
- Zhou L, McDonald C, Yawno T, Jenkin G, Miller S, Malhotra A. Umbilical cord blood and cord tissue-derived cell therapies for neonatal morbidities: current status and future challenges. Stem Cells Transl Med 2022;11(2):135–145. doi:10.1093/stcltm/szab024
- Zhou L, McDonald CA, Yawno T, et al. Feasibility of cord blood collection for autologous cell therapy applications in extremely preterm infants. Cytotherapy 2023;25(5):458–462. doi:10.1016/j.jcyt.2023.01.001
- Paton MCB, Wall DA, Elwood N, et al. Safety of allogeneic umbilical cord blood infusions for the treatment of neurological conditions: a systematic review of clinical studies. Cytotherapy 2022;24(1):2–9. doi:10.1016/j.jcyt.2021.07.001





Leave a Reply