Gestational trophoblastic disease (GTD) is a spectrum of disorders from benign to malignant, characterised by abnormal proliferation of trophoblastic tissue1 and affects approximately 1/1000 pregnancies. Given the rarity of the condition, it can be a very confronting and surprising diagnosis for a woman experiencing pregnancy loss.2 A number of internationally recognised guidelines now exist informing effective and patient-centred care, making this disease highly treatable.3 4 5
Benign gestational trophoblastic disease
Benign gestational trophoblastic disease can be divided into complete hydatidiform moles (CHM) or partial hydatidiform moles (PHM). Hydatidiform moles represent 90% of gestational trophoblastic disease.6 At the time of normal conception, it is presumed that mitochondrial DNA is maternal and nuclear DNA is equal parts maternal and paternal, one set of chromosomes being derived from each parent.7 A complete molar pregnancy is diploid and occurs when an empty ovum is fertilised by one sperm (~80%) or two sperm (~20%) leading to a karyotype of 46XX.8 9 10 11 In keeping with an empty ovum and no maternal DNA, a placenta is formed but no fetal parts develop. A PHM occurs when two sperm fertilise a single ovum. The karyotyping is usually triploid and 69 XXX or 69 XXY. Fetal parts will develop and cardiac activity can be seen.12 13
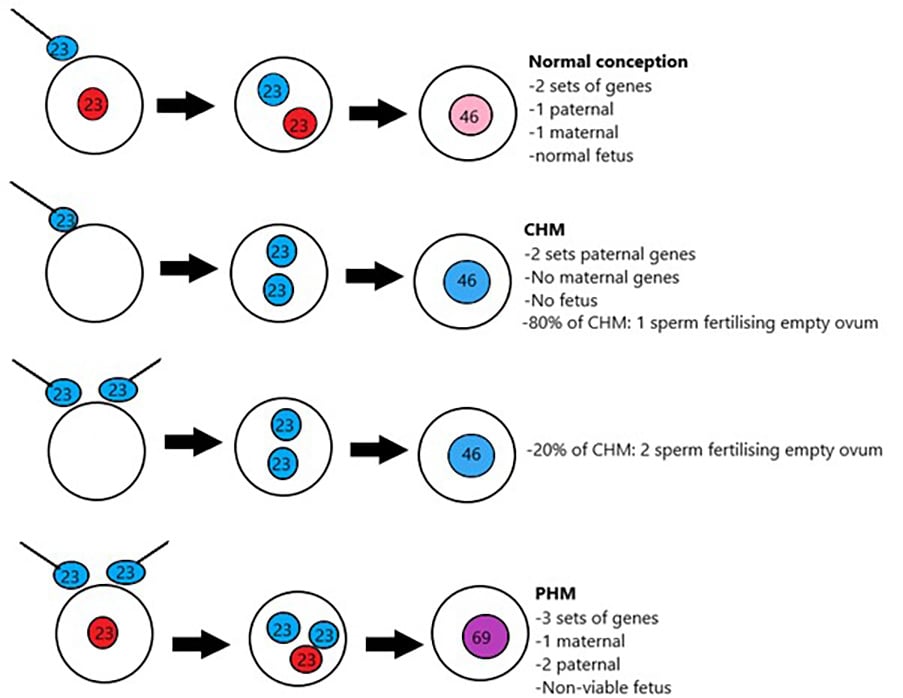
Figure 1. Molar pregnancy genetics.
Gestational trophoblastic neoplasia
Gestational trophoblastic neoplasia (GTN) includes the malignant gestational trophoblastic disease conditions of persistent trophoblastic disease, invasive mole, choriocarcinoma, placental site trophoblastic tumour (PSTT) and epithelioid trophoblastic tumour (ETT).14 Overall, GTN affects fewer than 1/40,000 pregnancies and the latter two conditions are extremely rare, affecting at most 1/50,000 pregnancies.15 GTN can be thought of as GTD that behaves in a malignant fashion, thus requiring further treatment in the form of chemotherapy and/or surgery when human Chorionic Gonadotrophin (hCG) levels are persistent, rising or metastatic disease is detected. Whilst 60% of GTN originates from molar pregnancies, 30% arises from previous miscarriage, normal delivery or abortion and a further 10% from ectopic pregnancies.16 17 18 19
Table 1. Comparing molar pregnancies.
| Benign Gestational Trophoblastic Disease | ||
| Type | Complete Hydatidiform Mole | Partial Hydatidiform Mole |
| Incidence | 1/1500 | 1/750 |
| Ploidy | Diploid | Usually triploid |
| Paternal DNA | 1 set (80%): 23X, which then duplicates to 46 XX
2 sets (20%): 23X +/- 23X or 23Y |
2 sets:
10% 23X |
| Maternal DNA | No maternal DNA | 1 set maternal DNA: 23X |
| Karyotype | 46 XX (most common) 46 XY |
69 XXY (most common) 69 XXX |
| Immunohistochemistry | P57 – | P57 + |
| Morphology (key examples) | Hydropic villi-diffuse
Trophoblastic hyperplasia- diffuse |
Partially cystic
Trophoblastic hyperplasia- focal Villous scalloping Stromal trophoblastic inclusions |
| Fetal tissue | Absent | Partially present |
| Presentation | Huge uterus
High hCG Medical complications:
|
Mistaken for miscarriage
Often minimal symptoms Vaginal bleeding in early pregnancy |
| Diagnosis | Clinical or ultrasound diagnosis
Post miscarriage histology (less common) |
Post miscarriage |
| Ultrasound findings | Theca lutein cysts
Snowstorm pattern Complex echogenic intrauterine mass |
Swiss cheese pattern of placental tissue
Formed fetus/ fetal parts Possible initial cardiac activity, followed by miscarriage |
| Malignancy / post molar gestational trophoblastic neoplasm (GTN) | 20% (majority local invasion)
Distant mets in ~5% |
0.5–5% |
Presentation
Vaginal bleeding, which is the most common first presentation of gestational trophoblastic disease, will often trigger an ultrasonographic evaluation.20 21 Classic ultrasound findings of CHM are an intrauterine snowstorm appearance and theca lutein cysts, but earlier and more advanced ultrasound assessment more commonly demonstrates a complex intra-uterine mass with no fetal parts and a cystic appearance of the placenta.22 The findings with PHM are often more subtle and initially may be mistaken on ultrasound as a miscarriage although some sonographic features might raise the suspicion (e.g. thickened hydropic placenta with or without a ‘Swiss cheese’ appearance). In many cases the diagnosis is only made once histopathological examination has taken place. A diagnosis of gestational trophoblastic disease should be considered in patients with risk factors such as extremes of maternal age and previous molar pregnancies.23 24
Historically, patients with CHM presented with an enlarged uterus, hyperemesis gravidarium, hypertension before 20 weeks gestation and hyperthyroidism. The current trend for early ultrasound assessment means that these clinical findings are rare; therefore, whilst careful consideration should be given to medical manifestations of the disease, gestational trophoblastic disease is typically diagnosed post evacuation of uterine contents.25 26
CHM have a malignant potential of 20% and a 5% chance of distant metastatic spread.27 28 PHM carry a malignant potential of 0.5–5%. 29 30 gestational trophoblastic disease management must therefore be timely and according to evidence-based guidelines. While recurrence rates for GTN are low at 0.6–2%, the risk of recurrence significantly increases without long-term follow up, supporting the need for ongoing care.31
Management
If gestational trophoblastic disease is suspected on ultrasound, serum hCG levels should be taken as well as patient blood type and a baseline full blood count. Liver function, thyroid function and coagulation profile can also be considered. If the diagnosis is suspected, suction curette should be undertaken by an experienced practitioner, preferably with ultrasound guidance to reduce the risk of perforation and leaving residual tissue. Clear communication with the operating team and anaesthetist is imperative due to high bleeding risk of patients with GTD. Consideration should be given for two units of packed red cells to be available and uterotonics such as ergometrine to be readily available in the operating theatre.32 33 34 If oxytocics are required, they should be administered after evacuation.35 Tissue should be sent for histopathology and ancillary testing (ploidy and p57 status) if GTD is suspected.36 In a Rhesus negative patient, anti-D immunisation postoperatively is best practice.37 If there are any clinical features suggestive of metastases, e.g. shortness of breath, a chest radiograph should be taken.38 Likewise, if the index of suspicion is high pre-operatively, weekly HCG monitoring post evacuation should be considered, even before histopathological confirmation.
Following confirmation of a CHM or PHM, it is essential that the patient is informed and advised of the importance of follow up, risk of persistent disease and the need to avoid further pregnancy until advised otherwise. In Australia there are two state based gestational trophoblastic disease registries in Victoria and Queensland.39 Clinicians in these states are encouraged to register patients after which ongoing monitoring and advice to patients is provided by the registry multidisciplinary teams.40 Outside of these states, care and follow up is provided by individual practitioners in accordance with RANZCOG guidelines. As well as registration, RANZCOG recommends following hCG levels in women weekly, commencing as soon as a diagnosis of a gestational trophoblastic disease is made. hCG levels are followed until three successive normal levels have been recorded. Following this, women with PHM can be discharged from follow up and those with CHM are recommended to have monthly levels for a further six months.41 42 43 44 In order to ensure consistency, hCG levels should preferably be carried out by the same laboratory with the request specifying ‘tumour’ hCG to encompass all possible hCG forms and thus avoid false negatives.45 Risk factors for post-molar GTN include initial hCG levels of >100,000, theca lutein cysts >6cm, an enlarged uterus and maternal age >40 years old.46
Histopathological features of gestational trophoblastic disease
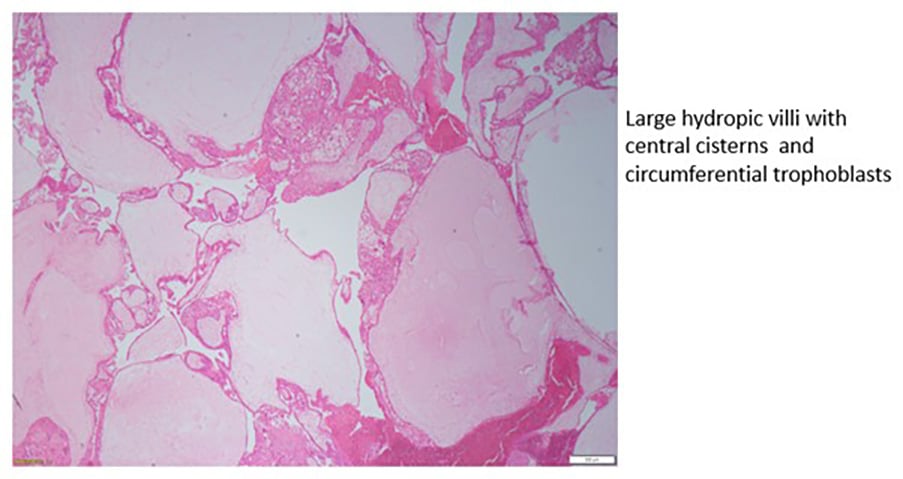
Figure 2a. Complete hydatidiform mole.
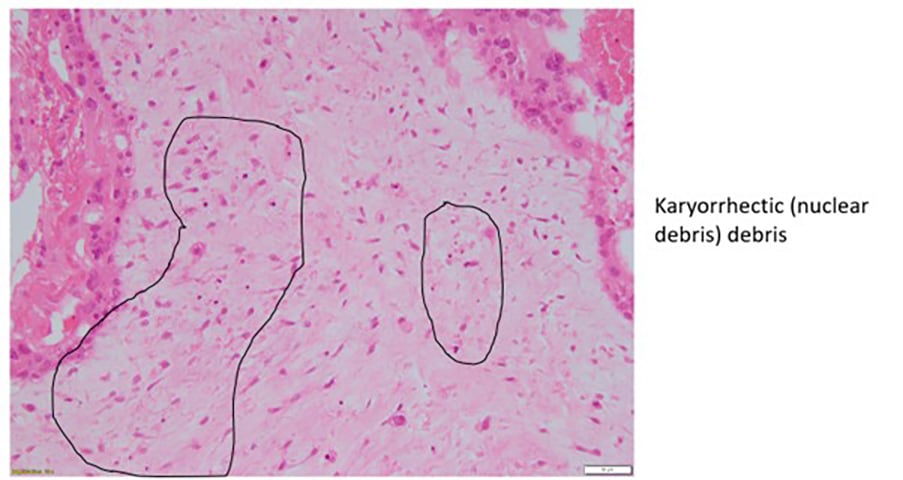
Figure 2b. Complete hydatidiform mole.
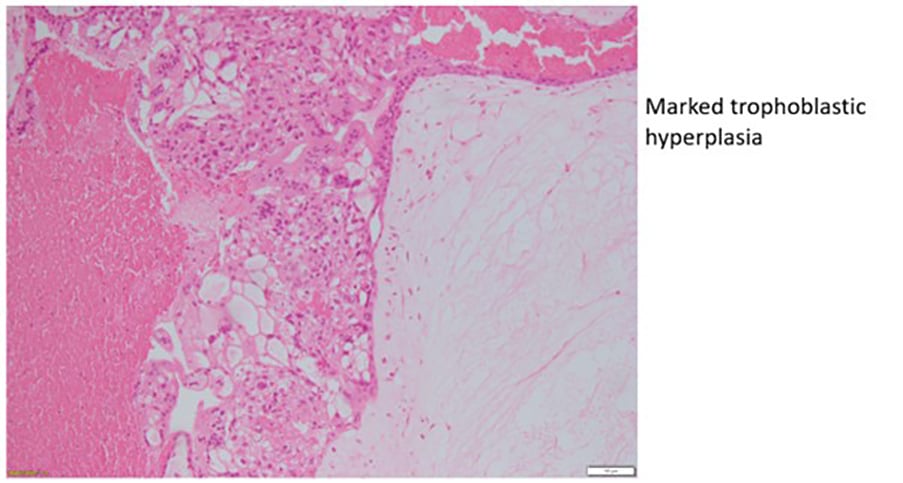
Figure 2c. Complete hydatidiform mole.
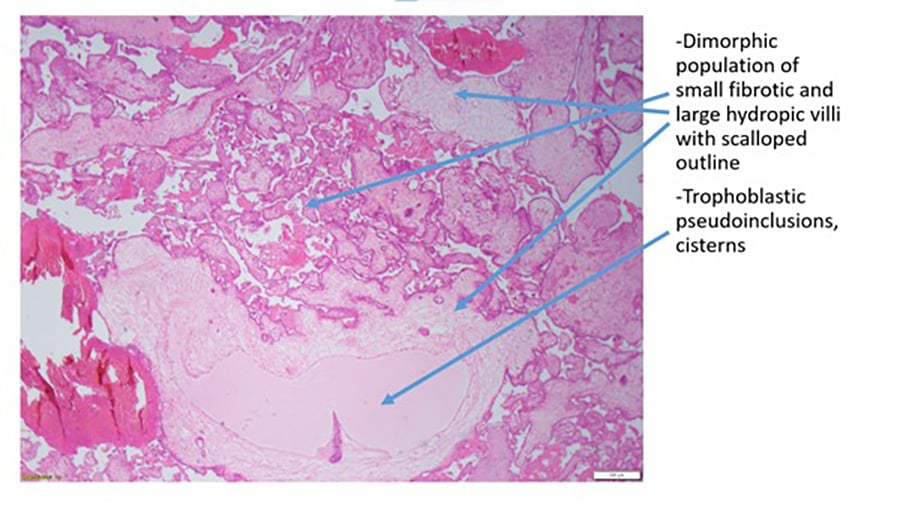
Figure 3a. Partial hydatidiform mole
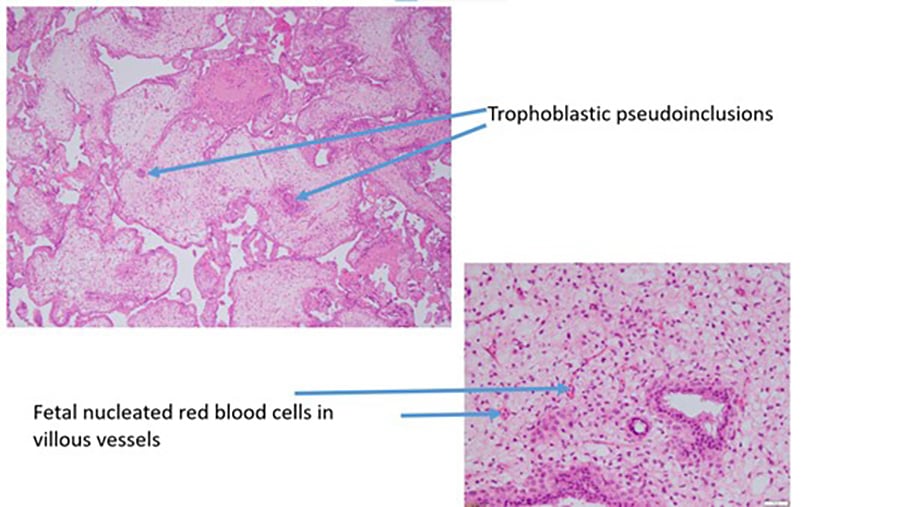
Figure 3b. Partial hydatidiform mole.
Staging of gestational trophoblastic neoplasia
Once a diagnosis of gestational trophoblastic neoplasia is made, the patient’s disease is assigned a FIGO stage and the WHO prognostic factors recorded (see figure 2c).47 48 49 50
Table 2. FIGO criteria for treatment of GTN.
| When to commence treatment |
| When the plateau of hCG lasts for four measurements over a period of three weeks or longer; that is, days 1, 7, 14, 21 |
| When there is a rise in hCG for three consecutive weekly measurements over at least a period of two weeks or more; days 1, 7, 14 |
| If there is a histologic diagnosis of choriocarcinoma |
After diagnosis, a thorough history and examination, serum hCG, a pelvic US and chest x-ray should be performed. The chest x-ray results and patient’s localising symptoms will guide further imaging requirements. If normal, no further imaging is required but if the possibility of metastases are raised, a CT chest should be arranged. Should this confirm chest metastases >1cm, an MRI brain with contrast as well as CT/MRI of the abdomen and pelvis is recommended.51 52
If the hCG is <5000 IU/L, and residual, non-myoinvasive, uterine confined disease is identified on ultrasound, a second curettage by an experienced practitioner can be considered. The patient should be counselled regarding risks of perforation, or brisk bleeding that might require further surgery (including hysterectomy) and that in up to 70% of cases, chemotherapy may still be required. 53 54 55
For women who do not desire future fertility, a hysterectomy can reduce the need for chemotherapy by up to 80%.56 The ovaries are normally preserved due to the rarity of ovarian involvement in GTN. If present, theca lutein cysts will generally resolve once hCG levels drop and their presence is not an indication for oophorectomy or cystectomy, irrespective of size, unless torsion is suspected. In cases of haemorrhagic bleeding and desire for fertility, uterine artery embolisation can be considered.57 The rare conditions PSTT and ETT usually need to be managed surgically due to their relative chemo-resistance.58 59 60 61
Response to chemotherapy is excellent for the vast majority of patients and the decision to use low- or high-risk regimens is based on FIGO stage and WHO risk score. 62 63 64 65 In general, in FIGO stage I disease a low-risk regimen is sufficient, FIGO stage IV requires a high-risk regimen and Stage II or III disease regimen depends on the WHO risk score.
There is nearly a 100% cure rate for low-risk patients (WHO score <7) with single agent chemotherapy, in the form of multi dose methotrexate (MTX) or Actinomycin D. MTX should be administered with folinic acid on proceeding days. 10–30% of low-risk patients will develop resistance to single agent chemotherapy.66 If hCG levels plateau or rise >10% over the course of two to four weeks of monitoring, resistance should be suspected. 15–20% of patients will require multi agent therapy. WHO Scores of 5–6 and/or choriocarcinoma may benefit from a higher risk regimen due to the higher risk of resistance to single agent chemotherapy.67
If the risk of recurrence is low (FIGO stage 1), MTX and Actinomycin D have equal efficacy and the choice of agent may be influenced by side effect profile and convenience/geography (four doses per fortnight in MTX versus a single Actinomycin dose fortnightly). Patients with stages II–III with a score of >7 or more and all stage IV are classified as having a high-risk disease recurrence and will require high-risk multiagent chemotherapy, the commonest regimen is EMACO (Etoposide, Methotrexate, Actinomycin D, Cyclophosphamide and Oncovin [Vincristine])68 69 in conjunction with a medical oncologist, preferably through a multidisciplinary gynae oncology team. Higher doses of MTX or intrathecal MTX to facilitate blood-brain barrier transport may also be required in managing brain metastases.70
Table 3. FIGO staging and classification for gestational trophoblastic neoplasia..
| FIGO stage | Description |
| I | Gestational trophoblastic tumours strictly confined to the uterine corpus |
| II | Gestational trophoblastic tumours extending to the adnexa or to the vagina, but limited to the genital structures |
| III | Gestational trophoblastic tumours extending to the lungs, with or without genital tract involvement |
| IV | All other metastatic sites |
Given GTN is exquisitely sensitive to chemotherapy, consideration should be given to a diagnosis of non-gestational choriocarcinoma (where a non-pregnancy related malignancy has de-differentiated into choriocarcinoma) if the response to chemotherapy is not as expected. In addition, if a large disease burden exists at the start of treatment (i.e. ‘ultra-high risk’ WHO score ≥12, brain metastasis, large lung lesions, etc), a gentle approach to chemotherapy might be advised to reduce the risk of sudden tumour necrosis or significant bleeding with subsequent risk of early patient death.71 72 73 74
Currently there is emerging data that immunotherapy (i.e. anti PD-1/PDL-1 immune check point inhibitors) could be effective in recurrent high-risk disease such as choriocarcinoma or ETT/PSTT.75
Table 4. WHO scoring system based on prognostic factors.
| WHO risk factor scoring with FIGO staging | 1 | 2 | 3 | 4 |
| Age | <40 | >40 | – | – |
| Antecedant pregnancy | Mole | Abortion | Term | – |
| Interval from index pregnancy | <4 months | 4–6 months | 7–12 months | >12 months |
| Pre-treatment hCG mIU/mL | <103 | >103–104 | >104–105 | >105 |
| Largest tumour size including uterus | – | 3–4cm | ≥5cm | – |
| Site of metastases including uterus | Lung | Spleen, kidney | Gastrointestinal tract | Brain, liver |
| Number of metastases identified | – | 1–4 | 5–8 | >8 |
| Previous failed chemotherapy | – | – | Single drug | Two or more drugs |
The future
The majority of recurrences (>85%) in those with high-risk GTN, occur in the first year.76 77 Patients should be counselled to avoid pregnancy for at least one year following any kind of GTN and treatment and support should be given to facilitate contraception choices. While the oral contraceptive pill was initially thought to slow the fall of hCG levels, the evidence for this is lacking and it is more important that women take a reliable form of contraceptive to prevent accidental pregnancy.
Once discharged from the registry after one year of follow up, 70% of women will go on to have a normal, term pregnancy. A woman can be reassured that the risk of gestational trophoblastic disease recurrence is only 1% in her subsequent pregnancy after a single molar pregnancy,78 79 but is closer to 25% if she has had two complete molar pregnancies.80 Women are therefore recommended to have an early ultrasound scan in any subsequent pregnancy to confirm a viable, non-molar pregnancy. A hCG level should be checked six weeks after every subsequent pregnancy, irrespective of outcome and consideration should be given to sending the placenta for histopathological evaluation.
Outcomes for patients with gestational trophoblastic disease have improved as a result of international consensus and evidence-based guidance.81 9 Any clinician caring for a woman with gestational trophoblastic disease should be aware of this guidance and have a low threshold to contact an experienced gestational trophoblastic disease centre should they have any concerns.
Our feature articles represent the views of our authors and do not necessarily represent the views of the Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG), who publish O&G Magazine. While we make every effort to ensure that the information we share is accurate, we welcome any comments, suggestions or correction of errors in our comments section below, or by emailing the editor at [email protected].
References
- Lok C, van Trommel N, Massuger L, et al. Clinical Working Party of the EOTTD. Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur J Cancer. 2020;130:228-240.
- RANZCOG. Management of gestational trophoblastic disease (C-Gyn 31). 2017. Available from: https://ranzcog.edu.au/wp-content/uploads/2022/05/Management-of-gestational-trophoblastic-disease.pdf
- Lok C, van Trommel N, Massuger L, et al. Clinical Working Party of the EOTTD. Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur J Cancer. 2020;130:228-240.
- RANZCOG. Management of gestational trophoblastic disease (C-Gyn 31). 2017. Available from: https://ranzcog.edu.au/wp-content/uploads/2022/05/Management-of-gestational-trophoblastic-disease.pdf
- Bruce S, Sorosky J. Gestational Trophoblastic Disease. Treasure Island, StatPearls Publishing. 2022. Available from: www.ncbi.nlm.nih.gov/books/NBK470267/
- Bruce S, Sorosky J. Gestational Trophoblastic Disease. Treasure Island, StatPearls Publishing. 2022. Available from: www.ncbi.nlm.nih.gov/books/NBK470267/
- Buza N, Hui P. Genotyping diagnosis of gestational trophoblastic disease: frontiers in precision medicine. Mod Pathol. 2021;34(9):1658-72.
- Bruce S, Sorosky J. Gestational Trophoblastic Disease. Treasure Island, StatPearls Publishing. 2022. Available from: www.ncbi.nlm.nih.gov/books/NBK470267/
- Buza N, Hui P. Genotyping diagnosis of gestational trophoblastic disease: frontiers in precision medicine. Mod Pathol. 2021;34(9):1658-72.
- Ngan HYS, Seckl MJ, Berkowitz RS, et al. On the diagnosis and management of gestational trophoblastic disease. Int J Gynaecol Obstet. 2018;143(Suppl 2):79-85.
- Ngan HYS, Seckl MJ, Berkowitz RS, et al. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynaecol Obstet. 2021;155(Suppl 1):86-93.
- Ngan HYS, Seckl MJ, Berkowitz RS, et al. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynaecol Obstet. 2021;155(Suppl 1):86-93.
- Cavoretto P, Cioffi R, Mangili G, et al. A Pictorial Ultrasound Essay of Gestational Trophoblastic Disease. J Ultrasound Med. 2020;39(3):597-613.
- Bruce S, Sorosky J. Gestational Trophoblastic Disease. Treasure Island, StatPearls Publishing. 2022. Available from: www.ncbi.nlm.nih.gov/books/NBK470267/
- Bruce S, Sorosky J. Gestational Trophoblastic Disease. Treasure Island, StatPearls Publishing. 2022. Available from: www.ncbi.nlm.nih.gov/books/NBK470267/
- Bruce S, Sorosky J. Gestational Trophoblastic Disease. Treasure Island, StatPearls Publishing. 2022. Available from: www.ncbi.nlm.nih.gov/books/NBK470267/
- Buza N, Hui P. Genotyping diagnosis of gestational trophoblastic disease: frontiers in precision medicine. Mod Pathol. 2021;34(9):1658-72.
- Ngan HYS, Seckl MJ, Berkowitz RS, et al. On the diagnosis and management of gestational trophoblastic disease. Int J Gynaecol Obstet. 2018;143(Suppl 2):79-85.
- Ngan HYS, Seckl MJ, Berkowitz RS, et al. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynaecol Obstet. 2021;155(Suppl 1):86-93.
- Ngan HYS, Seckl MJ, Berkowitz RS, et al. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynaecol Obstet. 2021;155(Suppl 1):86-93.
- Cavoretto P, Cioffi R, Mangili G, et al. A Pictorial Ultrasound Essay of Gestational Trophoblastic Disease. J Ultrasound Med. 2020;39(3):597-613.
- Cavoretto P, Cioffi R, Mangili G, et al. A Pictorial Ultrasound Essay of Gestational Trophoblastic Disease. J Ultrasound Med. 2020;39(3):597-613.
- Ngan HYS, Seckl MJ, Berkowitz RS, et al. On the diagnosis and management of gestational trophoblastic disease. Int J Gynaecol Obstet. 2018;143(Suppl 2):79-85.
- Ngan HYS, Seckl MJ, Berkowitz RS, et al. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynaecol Obstet. 2021;155(Suppl 1):86-93.
- Ngan HYS, Seckl MJ, Berkowitz RS, et al. On the diagnosis and management of gestational trophoblastic disease. Int J Gynaecol Obstet. 2018;143(Suppl 2):79-85.
- Ngan HYS, Seckl MJ, Berkowitz RS, et al. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynaecol Obstet. 2021;155(Suppl 1):86-93.
- Lok C, van Trommel N, Massuger L, et al. Clinical Working Party of the EOTTD. Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur J Cancer. 2020;130:228-240.
- Ngan HYS, Seckl MJ, Berkowitz RS, et al. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynaecol Obstet. 2021;155(Suppl 1):86-93.
- Lok C, van Trommel N, Massuger L, et al. Clinical Working Party of the EOTTD. Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur J Cancer. 2020;130:228-240.
- Ngan HYS, Seckl MJ, Berkowitz RS, et al. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynaecol Obstet. 2021;155(Suppl 1):86-93.
- Lok C, van Trommel N, Massuger L, et al. Clinical Working Party of the EOTTD. Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur J Cancer. 2020;130:228-240.
- Lok C, van Trommel N, Massuger L, et al. Clinical Working Party of the EOTTD. Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur J Cancer. 2020;130:228-240.
- Bruce S, Sorosky J. Gestational Trophoblastic Disease. Treasure Island, StatPearls Publishing. 2022. Available from: www.ncbi.nlm.nih.gov/books/NBK470267/
- Ngan HYS, Seckl MJ, Berkowitz RS, et al. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynaecol Obstet. 2021;155(Suppl 1):86-93.
- RANZCOG. Management of gestational trophoblastic disease (C-Gyn 31). 2017. Available from: https://ranzcog.edu.au/wp-content/uploads/2022/05/Management-of-gestational-trophoblastic-disease.pdf
- Lok C, van Trommel N, Massuger L, et al. Clinical Working Party of the EOTTD. Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur J Cancer. 2020;130:228-240.
- RANZCOG. Management of gestational trophoblastic disease (C-Gyn 31). 2017. Available from: https://ranzcog.edu.au/wp-content/uploads/2022/05/Management-of-gestational-trophoblastic-disease.pdf
- RANZCOG. Management of gestational trophoblastic disease (C-Gyn 31). 2017. Available from: https://ranzcog.edu.au/wp-content/uploads/2022/05/Management-of-gestational-trophoblastic-disease.pdf
- RANZCOG. Management of gestational trophoblastic disease (C-Gyn 31). 2017. Available from: https://ranzcog.edu.au/wp-content/uploads/2022/05/Management-of-gestational-trophoblastic-disease.pdf
- RANZCOG. Management of gestational trophoblastic disease (C-Gyn 31). 2017. Available from: https://ranzcog.edu.au/wp-content/uploads/2022/05/Management-of-gestational-trophoblastic-disease.pdf
- Lok C, van Trommel N, Massuger L, et al. Clinical Working Party of the EOTTD. Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur J Cancer. 2020;130:228-240.
- RANZCOG. Management of gestational trophoblastic disease (C-Gyn 31). 2017. Available from: https://ranzcog.edu.au/wp-content/uploads/2022/05/Management-of-gestational-trophoblastic-disease.pdf
- Bruce S, Sorosky J. Gestational Trophoblastic Disease. Treasure Island, StatPearls Publishing. 2022. Available from: www.ncbi.nlm.nih.gov/books/NBK470267/
- Ngan HYS, Seckl MJ, Berkowitz RS, et al. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynaecol Obstet. 2021;155(Suppl 1):86-93.
- RANZCOG. Management of gestational trophoblastic disease (C-Gyn 31). 2017. Available from: https://ranzcog.edu.au/wp-content/uploads/2022/05/Management-of-gestational-trophoblastic-disease.pdf
- Bruce S, Sorosky J. Gestational Trophoblastic Disease. Treasure Island, StatPearls Publishing. 2022. Available from: www.ncbi.nlm.nih.gov/books/NBK470267/
- Lok C, van Trommel N, Massuger L, et al. Clinical Working Party of the EOTTD. Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur J Cancer. 2020;130:228-240.
- RANZCOG. Management of gestational trophoblastic disease (C-Gyn 31). 2017. Available from: https://ranzcog.edu.au/wp-content/uploads/2022/05/Management-of-gestational-trophoblastic-disease.pdf
- Ngan HYS, Seckl MJ, Berkowitz RS, et al. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynaecol Obstet. 2021;155(Suppl 1):86-93.
- De Nola R, Schönauer LM, Fiore MG, et al. Management of placental site trophoblastic tumor: Two case reports. Medicine (Baltimore). 2018;97(48):e13439.
- Lok C, van Trommel N, Massuger L, et al. Clinical Working Party of the EOTTD. Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur J Cancer. 2020;130:228-240.
- Ngan HYS, Seckl MJ, Berkowitz RS, et al. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynaecol Obstet. 2021;155(Suppl 1):86-93.
- Lok C, van Trommel N, Massuger L, et al. Clinical Working Party of the EOTTD. Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur J Cancer. 2020;130:228-240.
- Ngan HYS, Seckl MJ, Berkowitz RS, et al. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynaecol Obstet. 2021;155(Suppl 1):86-93.
- Savage P. Seckl MJ. The role of repeat uterine evacuation in trophoblast disease [letter to the editor]. Gynaecol Oncol. 2005;99:251-2.
- Bolze PA, Riedl C, Massardier J, et al. Mortality rate of gestational trophoblastic neoplasia with a FIGO score of ≥13. Am J Obstet Gynecol. 2016;214(3):390.e1-8.
- Lok C, van Trommel N, Massuger L, et al. Clinical Working Party of the EOTTD. Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur J Cancer. 2020;130:228-240.
- Lok C, van Trommel N, Massuger L, et al. Clinical Working Party of the EOTTD. Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur J Cancer. 2020;130:228-240.
- Ngan HYS, Seckl MJ, Berkowitz RS, et al. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynaecol Obstet. 2021;155(Suppl 1):86-93.
- Tsakiridis I, Giouleka S, Kalogiannidis I, et al. Diagnosis and Management of Gestational Trophoblastic Disease: A Comparative Review of National and International Guidelines. Obstet Gynecol Surv. 2020;75(12):747-56.
- Bolze PA, Riedl C, Massardier J, et al. Mortality rate of gestational trophoblastic neoplasia with a FIGO score of ≥13. Am J Obstet Gynecol. 2016;214(3):390.e1-8.
- Lok C, van Trommel N, Massuger L, et al. Clinical Working Party of the EOTTD. Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur J Cancer. 2020;130:228-240.
- Ngan HYS, Seckl MJ, Berkowitz RS, et al. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynaecol Obstet. 2021;155(Suppl 1):86-93.
- Tsakiridis I, Giouleka S, Kalogiannidis I, et al. Diagnosis and Management of Gestational Trophoblastic Disease: A Comparative Review of National and International Guidelines. Obstet Gynecol Surv. 2020;75(12):747-56.
- Bolze PA, Riedl C, Massardier J, et al. Mortality rate of gestational trophoblastic neoplasia with a FIGO score of ≥13. Am J Obstet Gynecol. 2016;214(3):390.e1-8.
- De Nola R, Schönauer LM, Fiore MG, et al. Management of placental site trophoblastic tumor: Two case reports. Medicine (Baltimore). 2018;97(48):e13439.
- Lok C, van Trommel N, Massuger L, et al. Clinical Working Party of the EOTTD. Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur J Cancer. 2020;130:228-240.
- Lok C, van Trommel N, Massuger L, et al. Clinical Working Party of the EOTTD. Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur J Cancer. 2020;130:228-240.
- Bolze PA, Riedl C, Massardier J, et al. Mortality rate of gestational trophoblastic neoplasia with a FIGO score of ≥13. Am J Obstet Gynecol. 2016;214(3):390.e1-8.
- Lok C, van Trommel N, Massuger L, et al. Clinical Working Party of the EOTTD. Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur J Cancer. 2020;130:228-240.
- Lok C, van Trommel N, Massuger L, et al. Clinical Working Party of the EOTTD. Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur J Cancer. 2020;130:228-240.
- RANZCOG. Management of gestational trophoblastic disease (C-Gyn 31). 2017. Available from: https://ranzcog.edu.au/wp-content/uploads/2022/05/Management-of-gestational-trophoblastic-disease.pdf
- Ngan HYS, Seckl MJ, Berkowitz RS, et al. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynaecol Obstet. 2021;155(Suppl 1):86-93.
- Bolze PA, Riedl C, Massardier J, et al. Mortality rate of gestational trophoblastic neoplasia with a FIGO score of ≥13. Am J Obstet Gynecol. 2016;214(3):390.e1-8.
- Clark JJ, Slater S, Seckl MJ. Treatment of gestational trophoblastic disease in the 2020s. Curr Opin Obstet Gynecol. 2021;33(1):7-12.
- Lok C, van Trommel N, Massuger L, et al. Clinical Working Party of the EOTTD. Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur J Cancer. 2020;130:228-240.
- Ngan HYS, Seckl MJ, Berkowitz RS, et al. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynaecol Obstet. 2021;155(Suppl 1):86-93.
- Ngan HYS, Seckl MJ, Berkowitz RS, et al. On the diagnosis and management of gestational trophoblastic disease. Int J Gynaecol Obstet. 2018;143(Suppl 2):79-85.
- Ngan HYS, Seckl MJ, Berkowitz RS, et al. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynaecol Obstet. 2021;155(Suppl 1):86-93.
- Eagles N, Sebire NJ, Short D, et al. Risk of recurrent molar pregnancies following complete and partial hydatidiform moles. Hum Reprod. 2015;30(9):2055-63.
- Lok C, van Trommel N, Massuger L, et al. Clinical Working Party of the EOTTD. Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur J Cancer. 2020;130:228-240.







Leave a Reply