Ovarian cancer is a devastating diagnosis affecting 1 in 87 Australian women in their lifetime.1 Patients are often diagnosed at an advanced stage portending a poor prognosis. The five-year survival rate is 48%.2 Ovarian cancer is a heterogenous disease, comprised of different histological subtypes with distinct aetiologies and molecular profiles that can potentially be exploited by targeted treatment approaches. Recently, new advances in molecular sequencing have enabled more rapid and cost-effective profiling. This has come alongside the advent of PARP inhibitors which have revolutionised the treatment of ovarian cancer, improving progression-free survival (PFS) and potentially curing some women. This review will focus primarily on the implications of molecular profiling for the treatment of epithelial ovarian cancer.
Aetiology of ovarian cancer
Tumourigenesis may be caused by a wide range of genomic mutations, in combination with various non-genetic risk factors. The most common genetic causes of ovarian cancer are homologous recombination deficiency and mismatch repair.3
Homologous recombination deficiency (HRD)
Homologous recombination (HR) is the high-fidelity method employed by cells to repair double-stranded DNA breaks. In HRD, breaks are repaired using alternative error-prone techniques that lead to aberrant DNA, predisposing the tissue to tumour development.
The best-known ovarian cancer susceptibility genes, BRCA1 and BRCA2, code for proteins involved in homologous recombination DNA repair. Mutations in HR genes may be germline, inherited in an autosomal dominant fashion, or somatic, present only in the neoplastic tissue. BRCA mutations can be found in any histologic subtype of ovarian cancer but are most frequently found in high-grade serous ovarian carcinomas (HGSOC). Germline BRCA1 and BRCA2 mutations confer a lifetime risk of ovarian cancer of up to 49% and 21% respectively,4 hence the recommendation for risk-reducing bilateral salpingo-oophorectomy between the ages of 35–40 in BRCA1 mutation carriers and 40–45 in BRCA2.5 6
In Australia, germline BRCA mutations are found in 14.1% of ovarian cancer patients (17.1% in those with HGSOC), 44% of which have no reported family history of breast or ovarian cancer.7 A further 6–7% have a somatic mutation in BRCA1 or BRCA2.8 9 Additionally, HR pathway alterations have been documented in a further 25% of HGSOC,10 including HR pathway genes such as RAD51C, RAD51D and PALB2 (6–10%) and by epigenetic silencing of HR genes, such as methylation of BRCA1 (7–17%) or RAD51C promoters (1.5–3%).11 12
Mismatch repair deficiency
The second most common cause of epithelial ovarian cancer is due to mutations in the DNA mismatch repair (MMR) pathway, which account for another 10–15% of ovarian cancers.13 MMR is a method for recognising and removing mismatched base pairs during DNA replication in otherwise complementary paired DNA strands. Lynch syndrome (also known as hereditary non-polyposis colorectal cancer or HNPCC) is an example of an ovarian cancer susceptibility syndrome in which mismatch repair deficiency is the culprit. The four genes linked to Lynch syndrome, MLH1, MSH2, MSH6 and PMS2, produce DNA mismatch repair proteins. Mutations in these genes confer a lifetime risk of ovarian cancer of up to 17%.14
Molecular profiling in epithelial ovarian cancer
For women diagnosed with ovarian cancer, identification of specific genetic mutations has implications for treatment. Cancer Australia recommends that women newly diagnosed with invasive epithelial ovarian, fallopian tube or primary peritoneal cancer, regardless of their age or family history, should be offered assessment of their genetic risk.15 While mainstream genetic testing for BRCA1 and BRCA2 germline mutations has been integrated into routine cancer care for many women, integration with familial cancer clinics is necessary for all women with a positive result to discuss personal cancer risk and arrange cascade testing for relatives. Given the treatment implications, namely access to PARP inhibitors, somatic mutational testing is now also standard of care. Furthermore, although not standard in Australia, a range of assays referred to as ‘HRD tests’ have been developed to identify HRD cancers beyond those with BRCA mutations that may also be sensitive to PARP inhibitors.16
Next generation sequencing is a term for DNA sequencing technology introduced in the mid-2000s which allows for massively parallel DNA sequencing and hence rapid and economical tumour genomic profiling for individual patients. This has shifted the focus of genomic profiling from that of mainly risk assessment, to that of therapeutic planning. Alongside improvements in diagnostic tests have come multiple potential targeted therapies for ovarian cancer, including biomarker-driven treatments undertaken in the setting of a clinical trial.
Treatment for ovarian cancer
The cornerstone of treatment for ovarian cancer remains maximal cytoreductive surgery followed by chemotherapy. More recently, the importance of frontline maintenance therapy has been realised, with expanding indications for PARP inhibitor therapy, establishing a new standard of care for HRD ovarian cancers.
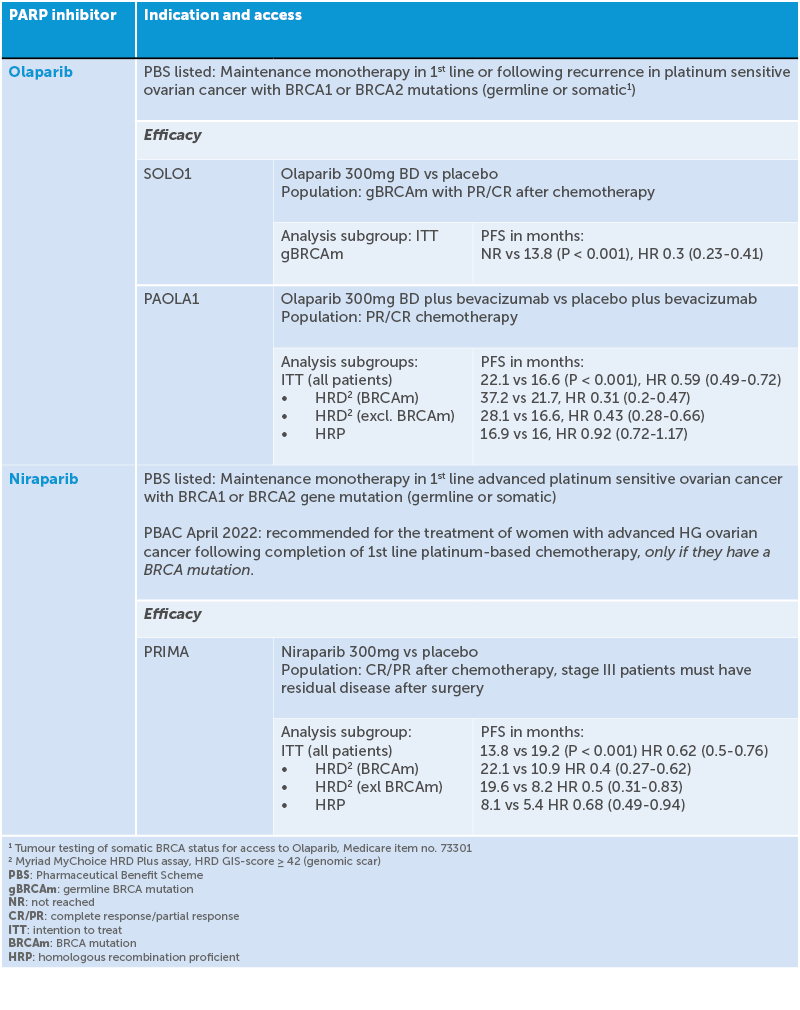
Since the 1990s, carboplatin-paclitaxel chemotherapy has remained the standard systemic treatment for ovarian cancer and is successful in shrinking tumour burden in 70–80% of patients. Unfortunately, up to 85% of patients with advanced ovarian cancer have a recurrence after completing chemotherapy.17 Ovarian cancers with BRCA gene mutations or other HRD have an increased platinum sensitivity and improved prognosis.18 They also show a superior response to PARP inhibitor therapy and maintenance PARP inhibitor use following platinum response has been shown to extend PFS.
Poly ADP-ribose polymerases (PARPs) are proteins that are key regulators of DNA damage repair processes. PARP inhibitors are a class of drugs that block the action of these proteins. When given to women with BRCA-mutated tumours, the resultant excessive accumulation of unrepaired DNA strand breaks leads to cancer cell death, a phenomenon called ‘synthetic lethality’. In 2019, three promising phase III studies of PARP inhibitors for upfront maintenance therapy in ovarian cancer – PRIMA19 VELIA20 and PAOLA-121 – were simultaneously published and altered the management of ovarian cancer. Four randomised double-blinded studies incorporating PARP inhibitors into frontline maintenance therapy have shown positive results, even suggesting cure,22 leading to FDA approval. SOLO-123 showed that patients with a BRCA mutation who received a PARP inhibitor following platinum response had an additional median 3.5 years free of disease progression and at five years 48% of patients were progression free compared to 21% with placebo. PRIMA24 found that among patients with newly diagnosed advanced ovarian cancer who responded to platinum-based chemotherapy, those who received the PARP inhibitor niraparib had significantly longer PFS than those who received placebo. This was true regardless of whether the patients had tumours with HRD or not, though the PFS was longer if they had HRD. PAOLA-125 found that in patients with advanced ovarian cancer receiving first-line standard therapy including bevacizumab, the addition of maintenance olaparib provided a significant PFS benefit, which was substantial in patients with HRD-positive tumours, including those without a BRCA mutation.
These studies have altered the way that maintenance therapy for ovarian cancer is prescribed and are improving the prognosis for women with ovarian cancer.
PARP inhibitors have improved the PFS in platinum sensitive tumours; however, 10–15% of tumours are platinum resistant at baseline and in those that recur following initial platinum response, resistance to platinum-based chemotherapy eventually arises. Attention is now being turned to targeting molecular changes unique to platinum resistant ovarian cancer.
Table 1. Indication and access of PARP inhibitors
Summary
Recent improvements in multipanel genetic testing, alongside targeted therapies exploiting tumour biomarkers, are being integrated into treatment guidelines for ovarian cancer. Every patient with ovarian cancer should be referred for genetic counselling and offered germline testing for BRCA mutations. Those without a germline genetic mutation should be offered somatic testing. Deleterious BRCA mutations and homologous recombination deficiency are now recognised as predictive biomarkers for the use of PARP inhibitors in women with ovarian cancer. Maintenance therapy with a PARP inhibitor is strongly recommended in women with these mutations.
Our feature articles represent the views of our authors and do not necessarily represent the views of the Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG), who publish O&G Magazine. While we make every effort to ensure that the information we share is accurate, we welcome any comments, suggestions or correction of errors in our comments section below, or by emailing the editor at [email protected].
References
- Australian Institute of Health and Welfare 2021. Cancer in Australia 2021. Cancer series no. 133. Canberra: AIHW : s.n., Cat no. CAN 144.
- Australian Institute of Health and Welfare 2021. Cancer in Australia 2021. Cancer series no. 133. Canberra: AIHW : s.n., Cat no. CAN 144.
- Haunschild CE, Tewari KS. The current landscape of molecular profiling in the treatment of epithelial ovarian cancer. Gynecologic Oncology. 2021;160:333-45.
- Kotsopoulos J, Gronwald J, Karlan B. Age-specific ovarian cancer risks among women with a BRCA1 or BRCA2 mutation. Gynecol Oncol. 2018;150:85-91.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Breast, Ovarian and Pancreatic. s.l. : NCCN, Version 2.2022.
- eviQ. BRCA1 or BRCA2 – risk management (female). Available from: www.eviq.org.au/cancer-genetics/adult/risk-management/3814-brca1-or-brca2-risk-management-female.
- Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: A report from the Australian Ovarian Cancer Study Group. Journal of Clinical Oncology. 2012;30:2654-63.
- Haunschild CE, Tewari KS. The current landscape of molecular profiling in the treatment of epithelial ovarian cancer. Gynecologic Oncology. 2021;160:333-45.
- The Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609-15.
- The Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609-15.
- Pennington K. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764-75.
- Norquist B. Inherited mutations in women with ovarian carcinoma. JAMA. 2016;2:482-90.
- Haunschild CE, Tewari KS. The current landscape of molecular profiling in the treatment of epithelial ovarian cancer. Gynecologic Oncology. 2021;160:333-45.
- Dominguez-Valentin M, Sampson JR, Seppala TT. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: findings form the Prospective Lynch Syndrome Database. Genetics in Medicine. 2020;2215-25.
- Online: canceraustralia.gov.au.
- Miller RE, Leary A, Scott CL, et al. ESMO recommendations on predictive biomarker testing for homologous recombination deficiency and PARP inhibitor benefit in ovarian cancer.. Annals of Oncology. 2020;31:1606-22.
- Gonzalez-Martin, A. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391-2402.
- Yang D, Khan S, Sun Y. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutation phenotype in patients with ovarian cancer. JAMA. 2011;306:1557-65.
- Gonzalez-Martin, A. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391-2402.
- Coleman RL. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403-15.
- Ray-Coquard I. Olaparib plus bevacizumab as first-line maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2416-28.
- Moore K. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Moore K. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Gonzalez-Martin, A. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391-2402.
- Ray-Coquard I. Olaparib plus bevacizumab as first-line maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2416-28.