Pulmonary embolism (PE) is the leading cause of maternal mortality in the developed world,1 and deep vein thrombosis (DVT) can lead to post-thrombotic syndrome, which is associated with a reduced quality of life. The incidence of VTE (venous thromboembolism, mainly comprising DVT and PE) in pregnancy is approximately 1–2 in 1000 pregnancies,2 3 with a risk that is static across the three trimesters; however, the daily absolute risk is highest in the postpartum period.4 Approximately two-thirds of lower-limb DVT occur in the antepartum period,5 with left-sided involvement in over 95% of cases;6 however, the majority of pregnancy-related PE occur in the postpartum period.7
Pregnancy is a hypercoagulable state, which is necessary to prepare the woman for the haemostatic challenge of delivery. Levels of fibrinogen and factors V, IX, X and VIII are increased, leading to enhanced thrombin generation, along with von Willebrand factor. Levels of the anticoagulant protein S is reduced, and pregnancy leads to a state of acquired protein C resistance.8 Venous stasis begins in the first trimester and peaks at 36 weeks gestation, due to a combination of oestrogen-mediated reduction in venous smooth muscle tone (leading to venous distension), pelvic venous compression by the gravid uterus and endothelial injury at the point of crossing/compression of the left iliac vein by the right iliac artery.9 10 The latter likely explains the striking propensity for involvement of the left leg veins by DVT. Endothelial damage occurring at the time of delivery likely accounts for the higher daily risk of VTE postpartum.11
Risk factors for pregnancy-related VTE include hereditary or acquired thrombophilia, personal or family history of VTE, superficial venous thrombosis, obesity, postpartum haemorrhage, caesarean delivery and assisted reproduction (Table 1).
Table 1. Risk Factors for pregnancy associated VTE.
| Risk factor | Odds ratio (95% CI) |
|---|---|
| Factor V Leiden (heterozygous) | 8.3 (5.4–12.7) |
| Factor V Leiden (homozygous) | 34.4 (9.9–120.1) |
| Antithrombin deficiency | 4.7 (1.3–16.9) |
| Protein C deficiency | 4.8 (2.2–10.6) |
| Protein S deficiency | 3.2 (1.5–6.9) |
| Antiphospholipid antibodies | 15.8 (10.9–22.8) |
| Previous VTE | 24.8 (17.1–36.0) |
| Family history of VTE | 3.9* |
| BMI >30mg/m2 | 5.3 (2.1–13.5) |
| Assisted reproduction | 4.3 (2.0–9.4) |
| Blood transfusion | 7.6 (6.2–9.4) |
| Antepartum haemorrhage | 2.3 (1.8–2.8) |
| Postpartum haemorrhage | 4.1 (2.3–7.3) |
| Pre-eclampsia | 3.1 (1.8–5.3) |
| IUGR | 3.8 (1.4–10.2) |
| Pre-eclampsia and IUGR | 5.8 (2.1–16.0) |
| Emergency caesarean delivery | 2.7 (1.8–4.1) |
*95% CI not reported
Data from Bourjeily et al,12 Chunilal & Bates,13 Greer14
Diagnosis
The threshold of suspicion for VTE in pregnancy is low, due to high maternal morbidity and mortality, and due to many symptoms of normal pregnancy mimicking those of VTE. The D-dimer rises steadily throughout pregnancy and often above the diagnostic cut-off threshold of 500mcg/L,15 and only a few studies have addressed clinical decision rules combined with D-dimer in pregnancy.
One such study evaluated 498 pregnant women with clinically suspected PE using three criteria from the YEARS algorithm (clinical signs of DVT, haemoptysis and PE as the most likely diagnosis).16 PE was ruled out if none of the three criteria were met and the D-dimer level was <1000ng/mL, or if one or more of the three criteria were met and the D-dimer level was <500ng/mL; amounting to a PE rule-out in 65% of patients in the first trimester and 32% in the third trimester. The VTE rate at three-month follow up was low at 0.21% (95% CI 0.04-1.2). However, this relies on the experience of the clinician when evaluating whether or not PE is the most likely diagnosis.
A recent meta-analysis of four studies showed that a negative D-dimer was highly sensitive to rule out VTE in pregnant women with suspected VTE and a disease prevalence consistent with a low/intermediate or unlikely pretest probability, with a high negative predictive value.17 However, the numbers included in the studies are small and further trials are needed to derive and validate specific clinical decision rules, and to determine the optimal D-dimer cut off during pregnancy.
The gold standard investigation for diagnosis of lower-limb DVT during pregnancy is complete compression ultrasound with visualisation of the iliac vein. If clinical suspicion remains despite a negative CUS, then MRI of the pelvic veins may be considered, although this technique is not validated for DVT.18 19
Figure 1 shows a suggested algorithm for diagnosis of PE in pregnancy. Imaging of the lungs for PE using CTPA or VQ scanning expose both the pregnant woman and her fetus to radiation. The radiation exposure to the fetus is lower for CTPA compared with VQ scanning, but negligible via both methods. However, exposure to the proliferating breast tissue of the woman is lower with a VQ scan.20 21 22 23 24 For this reason, many guidelines recommend VQ scanning as the preferred test in pregnant women with suspected PE. However, VQ scanning is not widely available, and breast shielding reduces breast radiation exposure from CTPA by >50%.25 26 If a woman is suspected of having PE but also has symptoms suggestive of DVT (unilateral leg pain and/or swelling), then a leg ultrasound should be performed and if a DVT is diagnosed, this obviates the need for lung scanning as the treatment is identical.
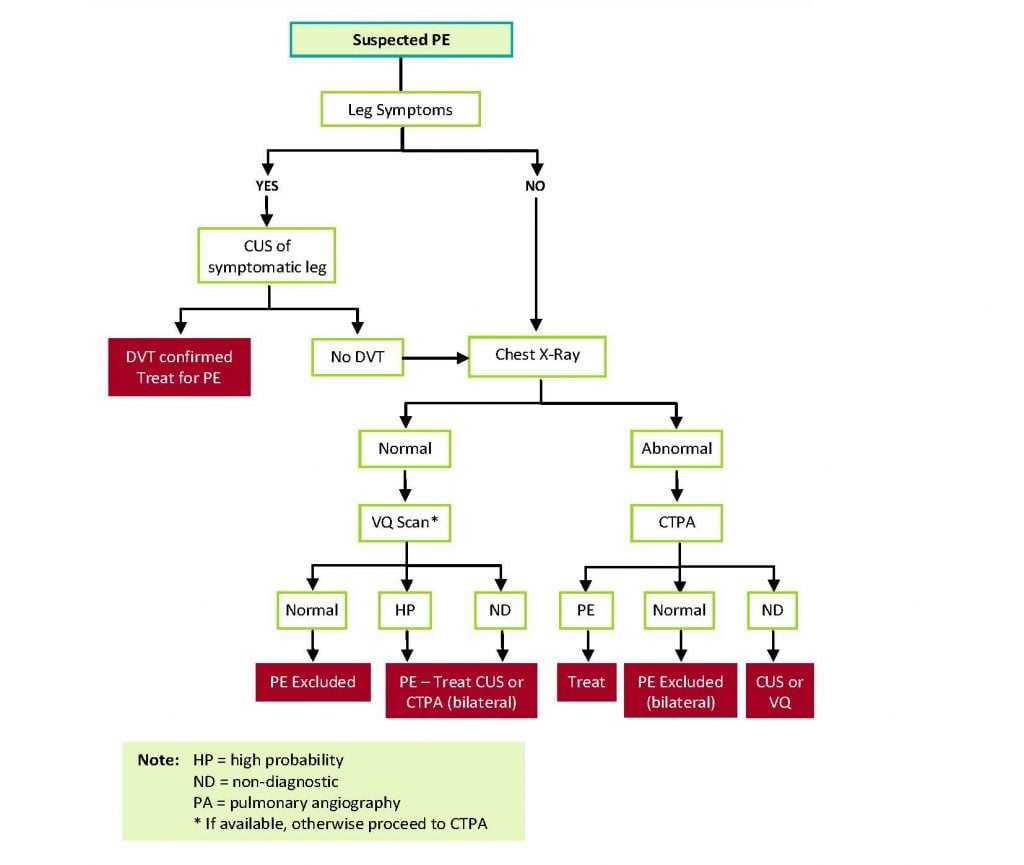
Figure 1. Diagnostic algorithm for PE in pregnancy.
Treatment
Low molecular weight heparin (LMWH) is the anticoagulant of choice for pregnant women, as LMWH does not cross the placenta and does not affect the developing fetus. Warfarin is teratogenic during pregnancy, but safe to use during breastfeeding. Direct oral anticoagulants (DOACs) are contraindicated in pregnancy and during breastfeeding due to limited human safety data and possible animal toxicity.27
For initial VTE treatment, there is no advantage in using a twice-daily LWMH regimen over once-daily, except in cases of extensive PE or DVT where a twice-daily regimen may be preferred for the first two weeks. It is usual to dose based on total body weight rather than pre-pregnancy weight. Monitoring with anti-Xa levels is not necessary except for women at extremes of body weight (<50kg or >90kg) or those with renal impairment (Cre Cl <30 ml/min) or recurrent VTE.28 In such cases, an anti-Xa peak level of 0.5-1.0 U/mL four hours post-injection is recommended.29
The anticoagulation should be continued throughout pregnancy and for at least six weeks postpartum, with a minimum of six months anticoagulation for acute proximal DVT/PE.30 A shorter total duration of therapy (6–8 weeks) may be appropriate in women with isolated distal (calf vein) DVT, with a possible reduction to prophylactic LMWH for the remainder of pregnancy and six-week postpartum period.31 A reduced intensity of anticoagulation after a period of full-dose treatment during pregnancy could be considered and has been safe in other patient populations;32 however, data from large-scale prospective studies in pregnant women is not available.
Thrombolysis should not be withheld in pregnant women who have life-threatening haemodynamic instability.33 A literature review on 23 cases of pregnant women receiving systemic thrombolysis for massive PE reported no maternal deaths, with bleeding complications in 39% of cases (major bleeding in 22%), with 9% of fetuses dying.34
Management of anticoagulants around time of delivery
For pregnant women receiving prophylactic doses of LMWH around the time of delivery, the ASH 2018 guideline35 suggests against scheduled delivery with discontinuation of prophylactic anticoagulation compared with allowing spontaneous labour. Women should be advised to stop LMWH at the first sign of contractions. For pregnant women that received therapeutic doses of LMWH around the time of delivery, the same guideline suggests scheduled delivery with prior discontinuation of anticoagulant therapy.
For women with VTE occurring more than three months prior to delivery, the last dose of 1mg/kg enoxaparin can be given 24 hours prior to a planned induction/caesarean section. For women with VTE occurring 1–3 months prior to delivery, the last dose of 1mg/kg enoxaparin can be given on the evening prior to induction; the patient can then be admitted the next morning and commenced on IV unfractionated heparin (IVUH) without a bolus. The heparin should be discontinued once the woman is in established labour (delivery anticipated within next 4–6 hours). The IVUH can be restarted 6–12 hours after delivery without a bolus (start at a low dose eg. 500 IU/hour) if haemostasis is adequate.
For VTE diagnosed within 1–3 weeks of delivery, a planned induction or caesarean section should be used as above with the use of IV heparin to allow the shortest time off anticoagulant. Placement of a retrievable IVC filter can be considered, especially within the first two weeks; however, the evidence base for these in pregnancy are limited and complications are potentially higher in this group.36
Conclusion
PE is the leading cause of maternal mortality in the developed world. Further trials are needed to derive/validate clinical decision rules, and to determine the optimal D-dimer cut-off in pregnancy. LMWH is the anticoagulant of choice for VTE prophylaxis and treatment during pregnancy; warfarin can safely be used postpartum. Delivery should be according to obstetric indications, with planned stopping and starting of anticoagulation in those on therapeutic anticoagulation.
Our feature articles represent the views of our authors and do not necessarily represent the views of the Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG), who publish O&G Magazine. While we make every effort to ensure that the information we share is accurate, we welcome any comments, suggestions or correction of errors in our comments section below, or by emailing the editor at [email protected].
Further reading
- Lee, et al. Low-Molecular-Weight-Heparin versus a Coumarin for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer. NEJM. 2003;349:146-53.
References
- Bourjeily, et al. Pulmonary embolism in pregnancy. Lancet. 2010;375:500-12.
- McLintock C, et al. Recommendations for the prevention of pregnancy-associated venous thromboembolism. ANZJOG. 2012;52(1):3-13.
- Middeldorp S, Ganzevoort W. How I treat venous thromboembolism in pregnancy. Blood. 2020;136(19):2133-42.
- Middeldorp S, Ganzevoort W. How I treat venous thromboembolism in pregnancy. Blood. 2020;136(19):2133-42.
- Middeldorp S, Ganzevoort W. How I treat venous thromboembolism in pregnancy. Blood. 2020;136(19):2133-42.
- Bourjeily, et al. Pulmonary embolism in pregnancy. Lancet. 2010;375:500-12.
- Middeldorp S, Ganzevoort W. How I treat venous thromboembolism in pregnancy. Blood. 2020;136(19):2133-42.
- Middeldorp S, Ganzevoort W. How I treat venous thromboembolism in pregnancy. Blood. 2020;136(19):2133-42.
- Middeldorp S, Ganzevoort W. How I treat venous thromboembolism in pregnancy. Blood. 2020;136(19):2133-42.
- Chunilal S, Bates S. Venous thromboembolism in pregnancy: diagnosis, management and prevention. Thromb Haemost. 2009;101:428-38.
- Chunilal S, Bates S. Venous thromboembolism in pregnancy: diagnosis, management and prevention. Thromb Haemost. 2009;101:428-38.
- Middeldorp S, Ganzevoort W. How I treat venous thromboembolism in pregnancy. Blood. 2020;136(19):2133-42.
- Chunilal S, Bates S. Venous thromboembolism in pregnancy: diagnosis, management and prevention. Thromb Haemost. 2009;101:428-38.
- Thrombosis in pregnancy: updates in diagnosis and management. Hematology Am Soc Hematol Edu Program. 2012:203-7.
- Bourjeily, et al. Pulmonary embolism in pregnancy. Lancet. 2010;375:500-12.
- Van der Pol L, et al. Pregnancy-adapted YEARS algorithm for diagnosis of suspected pulmonary embolism. NEJM. 2019;380:1139-49.
- Bellesini M, et al. D-dimer to rule out venous thromboembolism during pregnancy: A systematic review and meta-analysis. Thromb Haemost. 2021;19:2454-67.
- McLintock C, et al. Recommendations for the prevention of pregnancy-associated venous thromboembolism. ANZJOG. 2012;52(1):3-13.
- Middeldorp S, Ganzevoort W. How I treat venous thromboembolism in pregnancy. Blood. 2020;136(19):2133-42.
- Bourjeily, et al. Pulmonary embolism in pregnancy. Lancet. 2010;375:500-12.
- McLintock C, et al. Recommendations for the prevention of pregnancy-associated venous thromboembolism. ANZJOG. 2012;52(1):3-13.
- Middeldorp S, Ganzevoort W. How I treat venous thromboembolism in pregnancy. Blood. 2020;136(19):2133-42.
- Chunilal S, Bates S. Venous thromboembolism in pregnancy: diagnosis, management and prevention. Thromb Haemost. 2009;101:428-38.
- Thrombosis in pregnancy: updates in diagnosis and management. Hematology Am Soc Hematol Edu Program. 2012:203-7.
- Middeldorp S, Ganzevoort W. How I treat venous thromboembolism in pregnancy. Blood. 2020;136(19):2133-42.
- Chunilal S, Bates S. Venous thromboembolism in pregnancy: diagnosis, management and prevention. Thromb Haemost. 2009;101:428-38.
- Middeldorp S, Ganzevoort W. How I treat venous thromboembolism in pregnancy. Blood. 2020;136(19):2133-42.
- Thrombosis in pregnancy: updates in diagnosis and management. Hematology Am Soc Hematol Edu Program. 2012:203-7.
- McLintock C, et al. Recommendations for the prevention of pregnancy-associated venous thromboembolism. ANZJOG. 2012;52(1):3-13.
- Bellesini M, et al. D-dimer to rule out venous thromboembolism during pregnancy: A systematic review and meta-analysis. Thromb Haemost. 2021;19:2454-67.
- Bellesini M, et al. D-dimer to rule out venous thromboembolism during pregnancy: A systematic review and meta-analysis. Thromb Haemost. 2021;19:2454-67.
- Bates SM, et al. American Society of Haematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy. Blood Adv. 2018;2(22):3317-59.
- Bates SM, et al. American Society of Haematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy. Blood Adv. 2018;2(22):3317-59.
- Heavner MS, et al. Thrombolysis for Massive Pulmonary Embolism in Pregnancy. Pharmacotherapy. 2007;37(11);1449-57.
- Bates SM, et al. American Society of Haematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy. Blood Adv. 2018;2(22):3317-59.
- Crosby D, et al. Retrievable inferior vena cava filters in pregnancy: Risk versus benefit? Eur J Obstet Gynecol Reprod Biol. 2018;222:25-30.






Leave a Reply