Iron deficiency is the most common micronutrient deficiency worldwide, contributing significantly to the global burden of disease.1 Iron deficiency is poorly defined in the O&G setting, with variations in diagnostic thresholds, investigative frameworks and management strategies.2 3 Iron deficiency can occur with or without anaemia, and remains the most common cause of anaemia in pregnancy worldwide.4 Anaemia in pregnancy is defined by the World Health Organization as a haemoglobin (Hb) of <110g/L in the first 20 weeks, and <105g/L in the second 20 weeks of pregnancy.5 In this article, we aim to cover the essentials of iron deficiency for students and clinicians alike, with a focus on providing a framework for the investigation and management of iron deficiency in pregnancy and the early postpartum period.
Iron deficiency (ID) has important implications for both maternal and fetal health. For women of reproductive age, 14–22% have ID.6 Women with ID can experience impairment to muscle, immune and neurological function, and have lower reported quality of life.7 8 In Australia, 18% of pregnant people have iron deficiency anaemia (IDA), associated with a two- to threefold increase in iron requirements during pregnancy.9 10 This occurs largely due to an increase in maternal red cell volume and fetal erythropoiesis (see Figure 1).11 Inadequate iron stores are associated with immediate neonatal complications including preterm birth, low birth weight, and reduced iron stores in the newborn.12 13 In the longer term, ID in pregnancy has been associated with neurodevelopmental concerns, including cognitive and behavioural difficulties.14 Anaemia additionally has an impact on lactation, including reduced milk supply and lower rates of breastfeeding at six months.15 16
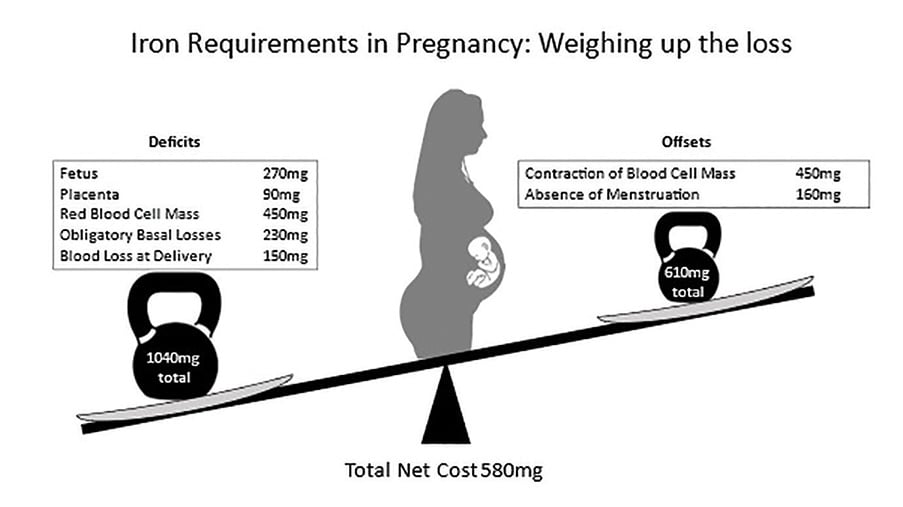
Figure 1. Iron requirements of pregnancy. Adapted from tables provided in reference 11.
Outside of pregnancy, heavy menstrual bleeding (HMB) is a common, under-recognised cause of ID in the O&G setting. It affects 25% of menstruating people, increasing in prevalence in the perimenopausal period.17 18ID in patients with HMB may go untreated as clinicians are focused on managing the HMB in isolation.19 Typically women experience >400 menstruations in their lifetime, depending on contraceptive and family choices, which can have a significant impact on blood loss and iron requirements.20 Historically, women experienced 100–150 menstruations over a lifetime due to increased parity; hence establishing amenorrhea to reduce overall iron losses may be a more physiological treatment option for those not wishing to conceive. It is important to recognise that neither HMB or pregnancy in isolation are likely to cause significant or refractory IDA, and further investigation is required in most cases.21 Guidance on the management of HMB can be found in the Australian Heavy Menstrual Bleeding Clinical Care Standard (2017).22
Interpretation of the full blood examination (FBE) and ferritin in pregnancy are challenging due to the normal reduction in some parameters due to physiological haemodilution. These changes are most significant from 28–36 weeks, which is similar to the timeframe of greatest iron requirement.23 It is important to recognise the difference between ID that has onset in later stages of pregnancy, where pregnancy factors are the likely cause, versus ID identified in the first trimester or non-pregnant state. We suggest checking ferritin levels as part of routine prenatal care. During preconception counselling, clinicians have an opportunity to discuss optimisation of iron status prior to pregnancy, and additionally identify and manage potential causes of ID. Of note, patients with ferritin levels >80ug/L in the first trimester are likely to have adequate iron stores for the remainder of their pregnancy.24
ID can be confirmed in pregnancy on the basis of a serum ferritin level <30ug/L. Complete iron studies are not typically required and are significantly more expensive (see Table 1). Serum iron levels have significant diurnal variation and can reflect recent iron intake. The FBE will confirm anaemia, and the red cell indices (MCV and MCH) and blood film can help confirm ID (ie. microcytic, hypochromic red cells), and distinguish from thalassaemia trait.25 Haemoglobinopathies, including thalassaemia and abnormal haemoglobins (eg. sickle cell disease), are increasing in prevalence in the Australian context due to immigration patterns.26 Occasionally, IDA is confused with thalassaemia trait as both conditions present with microcytic, hypochromic red cells, and it is possible for these conditions to co-occur. Individuals with anaemia in pregnancy due to thalassaemia trait do not require iron supplementation, unless they have proven co-existing iron deficiency.27
Table 1. Cost of pathological investigations as per MBS schedule.
Test |
Cost ($) |
| Full blood examination | 7.85 |
| Iron studies | 32.55 |
| Ferritin | 18.88 |
| Coeliac serology | 49.50 |
| Coeliac gene testing | 118.85 |
| B12 total/Active | 23.60/42.95 |
| Folate | 23.60 |
| Haemoglobin electrophoresis/chromotography | 90.20 |
| Alpha/Beta Thalassaemia Gene Analysis | 100–1000 |
Where ID is identified, particularly where refractory to iron replacement, potential causes should be carefully considered. A diet history is essential as approximately 40% of Australian women of reproductive age do not have adequate dietary iron intake.28 In premenopausal women with ID, British guidelines (endorsed by the gastroenterological society of Australia) recommend screening for Coeliac disease (CD), as this cohort of patients represent 4% of ID cases and are typically refractory to oral iron supplementation.29 30 31 In Australia, the prevalence of CD is approximately 1.4%, with a significant portion undiagnosed. Dietary gluten must remain for accurate testing via serology.32 HLA gene testing on gluten-free patients may assist as 99.6% of the CD population have genes coding HLA-DQ2 or HLA-DQ8; thus, a negative test largely excludes the possibility of CD.33 Identifying CD as a cause of ID in patients is important, as it has implications for fertility including recurrent miscarriage.34 Those at risk of mixed nutritional deficiency should have B12 and folate levels assessed. This includes patients with restrictive intake, malabsorption or refugee background where there is emerging B12 and folate deficiency.35 36
Recommended approach to managing iron deficiency in obstetric patients
Dietary sources are inadequate to replace iron stores for pregnant people with established ID, hence first-line therapy is oral iron supplementation. The Australian recommendations are to commence treatment where ferritin levels are <30ug/L.37 Recent data indicates that second daily iron dosing may be superior to daily dosing, with fewer side effects, and a higher total and fractional iron absorption.38 Whilst 100mg iron supplementation has a greater fractional absorption compared to 200mg, the total absorption is greater with 200mg, and as such would be advisable in IDA. Consideration should be given to lower doses (minimum 60mg elemental iron) for patients that are not anaemic.39 We suggest a trial of iron for four weeks, followed by repeat FBE and ferritin level. An earlier FBE at two weeks may be prudent in the setting of significant anaemia in early pregnancy, concerns regarding ongoing blood loss and/or uncertainty about the diagnosis. Treatment compliance should be reviewed on an ongoing basis, with consideration being given to missed doses and optimising tolerability (see Table 2). In anaemic patients, failure of oral therapy should be demonstrated by a lack of Hb response after four weeks of therapy. Pregnant people with Hb <70g/L or those who are hemodynamically unstable require prompt review and urgent referral to a Haematologist. Lifeblood’s flowchart ‘Hb assessment and optimisation in pregnancy’ is a useful management guideline.40
Table 2. Management options for side effects of oral iron therapy.
Side effect |
Intervention |
| Nausea/vomiting | Divided dosing, alternate formulation (eg. liquid, anti-emetics) |
| Constipation | Divided dosing, aperients |
| Teeth staining | Avoid liquid formulation |
| Reflux | Divided dosing, PPI therapy |
In later stages of pregnancy, where there are significant bleeding risks or where oral iron has been inadequate/not tolerated, intravenous (IV) iron replacement may be more appropriate. IV iron and its indications have been previously discussed in O&G Magazine along with potential side effects.41 Hypophosphatemia is a potential serious side effect of treatment with ferric carboxymaltose; however, is less well recognised compared to anaphylaxis, extravasation, and delayed serum sickness.42 Clinicians should be familiar with all potential side effects, and refer to their local guidelines prior to administering IV iron. The need for a blood transfusion should be considered based on clinical symptoms and a patient’s Hb level, and is generally appropriate in the postpartum setting where the Hb is <70g/L. When transfusion is indicated, a single red cell unit should be transfused with re-evaluation of the Hb and clinical status, unless clinically significant haemorrhage. The risk of transfusion and the potential for red cell alloimmunisation should be balanced against the potential of minimal benefit. Iron infusions may be given in addition to blood transfusion where additional iron is needed to support post pregnancy recovery, including for lactation. Of note, a single unit of red cells typically contains 250mg of iron. Lifeblood guidelines recommend oral iron therapy in the postpartum setting if Hb >80g/L in the non-transfused population for a minimum of six weeks.43
For all pregnant people, and those trying to conceive, it is essential to discuss dietary contributions to iron deficiency. Early identification of patients at risk of ID is important to facilitate a preventative rather than curative strategy. Key risk factors for ID are outlined in Table 3, and particular attention should be paid to patients with multiple risk factors. These patients should be considered for referral to an accredited practising dietitian. Dietary iron is differentiated as haem and non-haem, with haem iron having the greatest bioavailability (see examples of each in Table 4). Consider dietary intake of substances that inhibit or promote iron absorption. Phytates, including unprocessed bran, polyphenols including tea and coffee, and calcium decrease the absorption of non-haem iron particularly and should be spaced apart.44 In contrast, ascorbic acid found in foods such as citrus fruits, berries, broccoli, tomato and capsicum increases non-haem iron absorption, as does co-ingestion of haem iron foods. The possibility that an individual’s microbiome may lead to variations in iron absorption is the subject of ongoing research.
Table 3. Key factors to consider when screening patients at risk of iron deficiency.
Factors |
Examples |
| Does the patient have increased iron requirements? |
|
| Does the patient follow a diet reliant on non-haem iron sources? |
|
| Could the patient have impaired absorption of iron? |
|
| Does the patient have any other nutrition impact symptoms? |
|
Table 4. Examples of dietary sources of haem and non-haem iron.
Haem |
Non-haem |
| Red meat (eg. lamb, beef and pork) | Legumes |
| Poultry (eg. chicken, turkey) | Nuts (including nut butters) |
| Fish | Green leafy vegetables |
| Offal | Iron fortified cereals |
Table 5. The dos and don’ts of iron deficiency in pregnancy.
Do |
Don’t |
| Give clear dietary advice to all patients trying to conceive or who are already pregnant | Attempt to treat iron deficiency by changing diet alone |
| Utilise oral iron supplements as first-line treatment for iron deficiency | Forget to check for side effects of oral iron supplements |
| Check FBE and ferritin four weeks post initiating treatment | Routinely order full iron studies in pregnancy |
| Consider screening for Coeliac disease | Forget to consider second daily oral iron supplementation |
| Screen for Thalassemia when there are microcytic, hypochromic red cell indices and a normal ferritin | Forget your local guideline for IV iron and potential side effects including hypophosphatemia |
| Check B12 and folate for mixed nutritional deficiency where appropriate | Presume it’s not your job if it’s not an obstetric cause |
Conclusions
ID is a treatable cause of anaemia in pregnancy. We suggest clinicians focus on optimising iron stores prenatally where possible by providing clear dietary advice, and commencing oral iron supplementation where serum ferritin levels are low. For those who are already pregnant, second daily oral iron should be considered to improve both absorption and tolerability. In cases where there is refractory iron deficiency, or perceived higher risks of anaemia, IV iron replacement may be more appropriate. In all cases, ongoing review for treatment compliance and monitoring for the common side effects of oral iron replacement should be undertaken. Screening for common causes of ID in premenopausal people, including HMB and CD, may help to improve not only a patient’s iron status, but also their pregnancy outcome and overall quality of life.
Our feature articles represent the views of our authors and do not necessarily represent the views of the Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG), who publish O&G Magazine. While we make every effort to ensure that the information we share is accurate, we welcome any comments, suggestions or correction of errors in our comments section below, or by emailing the editor at [email protected].
References
- Bailey RL, West KP, Black RE. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab. 2015;66(suppl 2):22-33.
- Daru J, Allotey J, Pena-Rosas JP, et al. Serum ferritin thresholds for the diagnosis of iron deficiency in pregnancy: a systematic review. Transfus Med. 2017;27(3):167-74.
- Stoffel NU, Zeder C, Brittenham GM, et al. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron deficient anaemic women. Haematologica. 2020;105(5):1232-9.
- Juul SE, Derman RJ, Auerbach M. Perinatal iron deficiency: implications for mothers and infants. Neonatology. 2019;115(3):269-74.
- WHO. Iron Deficiency Anaemia, Assessment, Prevention, and Control: a Guide for Programme Managers. Geneva: World Health Organization. 2001.
- Stevens, GA, Finucane MM, De-Regil LM, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995-2011: a systematic analysis of population representative data. Lancet Glob Health. 2013;1(1):e16-25.
- Karlsson TS, Marions LB, Edlund MG. Heavy menstrual bleeding significantly affects quality of life. Acta obstet gynecol scand. 2014;93(1):52-7
- Fraser IS, Mansour D, Breymann C, et al. Prevalence of heavy menstrual bleeding and experiences of affected women in a European patient survey. Int J Gynaecol Obstet. 2015;128(3):196-200.
- Khalafallah A, Dennis A, Bates J, et al. A prospective randomized controlled trial of intravenous versus oral iron for moderate iron deficiency anaemia of pregnancy. J Intern Med. 2010;268(3):286-95.
- Soma-Pillay P, Nelson-Piercy C, Tolppanen H, et al. Physiological changes in pregnancy. Cardiovasc J Afr. 2016;27(2):89-94.
- Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr. 2000;72(1 suppl):257S-264S.
- Juul SE, Derman RJ, Auerbach M. Perinatal iron deficiency: implications for mothers and infants. Neonatology. 2019;115(3):269-74.
- Aub-ouf NM, Jan MM. The impact of maternal iron deficiency and iron deficiency anaemia on child’s health. Saudi Med J. 2015;36(2):146-9.
- Juul SE, Derman RJ, Auerbach M. Perinatal iron deficiency: implications for mothers and infants. Neonatology. 2019;115(3):269-74.
- Henley SJ, Anderson CM, Avery MD. Anaemia and insufficient breast milk in first-time mothers. Birth. 1995;22(2):86-92.
- Chua S, Gupta S, Curnow J, et al. Intravenous iron vs blood for acute post partum anaemia (IIBAPPA): a prospective randomisted trial. BMC Pregnancy and Childbirth. 2017;17(1)424.
- Karlsson TS, Marions LB, Edlund MG. Heavy menstrual bleeding significantly affects quality of life. Acta obstet gynecol scand. 2014;93(1):52-7
- Royal College of Obstetricians and Gynaecologists. National heavy menstrual bleeding audit. London: RCOG; 2014.
- Mansour D, Hofmann A, Gemzell-Danielsson. A review of the clinical guidelines on management of iron deficiency and iron deficiency anaemia in women with heavy menstrual bleeding. Adv Ther. 2021;38(1):201-25.
- Strassman B. The biology of menstruation in homo sapiens: total lifetime menses, fecundity and nonsynchrony in a natural fertility population. Curr Anthropol. 1997;38(1):123-9.
- Sinclair M. Iron deficiency: clinical update [internet].2nd ed. Mulgrave (AU): Gastroenterology Society of Australia; 2015.
- Australian Commission on Safety and Quality in Health Care. Heavy Menstrual Bleeding Clinical Care Standard. Sydney: Australian Commission on Safety and Quality in Health Care; 2017.
- Soma-Pillay P, Nelson-Piercy C, Tolppanen H, et al. Physiological changes in pregnancy. Cardiovasc J Afr. 2016;27(2):89-94.
- Hallberg L, Bengtsson C, Lapidus L, et al. Screening for iron deficiency: an analydis based on bone marrow examinations and serum ferritin determinations in a population of sample women. Br J Haematol. 1993;47(120):875-9.
- Van Vranken M. Evaluation if microcytosis. Am Fam Physician. 2010;82(9):1117-22.
- Tan YL, Kidson-Gerber G. Antenatal haemoglobinopathy screening in Australia. Med J Aust. 2016;203(6):226-30.
- Verma S, Gupta R, Kudesia M, et al. Coexisting iron deficiency anaemia and Beta thalassemia trait: effect of iron therapy on red cell parameters and hemoglobin subtypes. ISRN Hematol. 2014;2014:e1-5.
- Australian Bureau of Statistics. Australian health survey: usual nutritional intakes. Belconnen (AU): Australian Bureau of Statistics; 2015:44.
- Snook J, Bhala N, Beales ILP, et al. British society of gastroenterology guidelines for the management of iron deficiency anaemia in adults. Gut. 2021;70(11):2030-51.
- Hershko C, Camaschella C. How I treat unexplained refractory iron deficiency anaemia. Blood. 2014;123(3):326-33.
- Tye-Din J. Interpreting tests for coeliac disease: tips, pitfalls and updates. Aust J Gen Pract. 2018;47(1-2):28-33.
- Tye-Din J. Interpreting tests for coeliac disease: tips, pitfalls and updates. Aust J Gen Pract. 2018;47(1-2):28-33.
- Tye-Din J. Interpreting tests for coeliac disease: tips, pitfalls and updates. Aust J Gen Pract. 2018;47(1-2):28-33.
- Martinelli P, Troncone R, Paparo F, et al. Coeliac disease and unfavourable outcome of pregnancy. Gut. 2000;46(3):332-5.
- Benson J, Phillips C, Kay M, et al. Low vitamin B12 levels among newly-arrived refugees from Bhutan, Iran and Afghanistan: a multicentre Australian study. PLoS one. 2013;8(2):e57998.
- Brown RD, Langshaw MR, Uhr EJ, et al. The impact of mandatory fortification of flour with folic acid on the blood folate levels of an Australian population. Med J Aust. 2011;194(2):65-7.
- Australian Red Cross Lifeblood. Haemoglobin assessment and optimization in maternity. Melbourne: Australian Red Cross Lifeblood. 2020.
- Stoffel NU, Zeder C, Brittenham GM, et al. Iron absorption from supplements is greater with alternate day than consecutive day dosing in iron-deficient anaemic women. Haematologica. 2020;105(5):1232-9.
- Australian Red Cross Lifeblood. Haemoglobin assessment and optimization in maternity. Melbourne: Australian Red Cross Lifeblood. 2020.
- Australian Red Cross Lifeblood. Haemoglobin assessment and optimization in maternity. Melbourne: Australian Red Cross Lifeblood. 2020.
- Cutts B. Q&A: Iron infusions in pregnancy. O&G Magazine (RANZCOG). 2016;18(2). Available from: www.ogmagazine.org.au/18/2-18/qa-2/
- Ifie E, Oyibo S, Joshi H, et al. Symptomatic hypophosphataemia after intravenous iron therapy: an underrated adverse reaction. Endocrinol Diabetes Metab Case Rep. 2019(1):19-0065.
- Australian Red Cross Lifeblood. Haemoglobin assessment and optimization in maternity. Melbourne: Australian Red Cross Lifeblood. 2020.
- Hurrell R, Egli I. Iron bioavailability and dietary reference values. Am J Clin Nutr. 2010;91(5): 1461S-1467S.
- National Health and Medical Research Council [NHMRC]. Nutrient Reference Values for Australia and New Zealand: Iron. 2006 [updated 2014]. Available from: www.nrv.gov.au/nutrients/iron







Leave a Reply