Governance of assisted reproductive treatment (ART) and surrogacy in Australia and New Zealand (NZ) includes a complex mix of ethical guidelines and legislation. In Australia, New South Wales (NSW), Victoria, South Australia (SA), and Western Australia (WA) have legislation regulating ART and all states and territories except the Northern Territory (NT), have legislation governing surrogacy. Clinics must also adhere to the National Health and Medical Research Council Ethical guidelines on the use of assisted reproductive technology in clinical practice and research 2017,1 and self-regulatory requirements established by the Fertility Society of Australia (FSA) and its Reproductive Technology Accreditation Committee (RTAC). In NZ, ART and surrogacy are governed via the Human Assisted Reproductive Technology Act 2004 and guidelines issued by the Advisory Committee on Assisted Reproduction.
ART and surrogacy must be ‘conducted in a manner that shows respect, minimises potential harms and supports the ongoing wellbeing of all parties, including persons born as a result of ART’.2 This recognises that ART involving donor and surrogacy (with or without donor) may lead to complex biological and/or social relations, in which there is a need for exchange of information and possibly, contact between donors, recipients, persons born and surrogates. It also aims to prevent exploitation and commodification of individuals participating in donor or surrogacy arrangements.
Gamete/embryo donation and surrogacy is altruistic only in Australia and NZ, but reimbursement of out-of-pocket expenses enforced, which may include medical, counselling, legal, childcare, travel or accommodation costs, loss of income, or costs associated with health, disability or life insurance that would have otherwise not been obtained. International donation of gametes may occur subject to local regulatory requirements.
The Reproductive Technology Accreditation Committee (RTAC) require donors of gametes and embryos to be screened for infectious diseases, family and personal medical issues and lifestyle issues. Sperm donors need to be between 21 and 50 years old with a normal semen analysis; oocyte donors need to preferably be between 21 and 35 years old and have completed their family.
Donor gamete/embryo
Access to Information
Donors of gametes may be known or unknown to the recipient. When unknown the donor must nevertheless be ‘open identity’ to enable access to information about biological heritage and relations.
While disclosure of donor-conception status is encouraged, it is not mandated in any jurisdiction. Some donor-conceived people report open disclosure within their families from an early age, while others report finding out accidently via DNA testing, during family breakdown, or as a result of illness (their own or family). Victoria is the only state to include an addendum to the birth certificate which informs the relevant person at age 18 that there is more information held on the birth register about them at their request. This acts as an incentive for recipient parents for early disclosure.
NZ, NSW, Victoria, and WA operate mandatory registries of donors and donor-conceived persons which record and release information about biological heritage and relations. In NZ, Māori donors must include additional detail on tribe and ancestry. Only Victoria enables access to information regardless of when the donation took place (including regarding donors who may once have thought their donation anonymous); while contact is subject to the donor’s consent. In the other jurisdictions, the registers act prospectively, access to information permitted for all donations after the law came into effect (NZ: 2005, NSW: 2010, WA: 2004). Release of information to the donor- conceived person is at the age of 18 (16 in WA) or recipient parents if younger than 18. NSW and WA also have voluntary registries for access prior to these dates. Donors can access limited non-identifying information about persons born from their donation.
In other states and territories, access may occur via requests to clinics, noting the NHMRC Guidelines have stipulated since 2004 that all donations must include consent to information release. SA is in the process of establishing a central donor register.
Increasingly, people born as a result of donor-conception are accessing direct-to-consumer DNA testing to find their biological heritage and relations.
Family limits
The number of families that may result from the use of a donor is limited, including previous/current spouse or families of donor, to:
- NSW: 5 women
- Vic: 10 women
- WA: 5 families worldwide
- SA, NT, Qld, ACT, Tas: (no legislation therefore RTAC guidance): 10 families worldwide
The donor is able to stipulate lower limits than these.
Surrogacy
Altruistic surrogacy is legal in Australia and NZ. A child born to a surrogate mother, with or without a partner, is considered their legal child until a parentage order or adoption is granted following birth, requiring consent from all parties and the court agreeing that this is in the child’s best interest. Surrogacy agreements are not enforceable, meaning commissioning parents or surrogate couple can change their mind during the surrogacy and after the child is born.
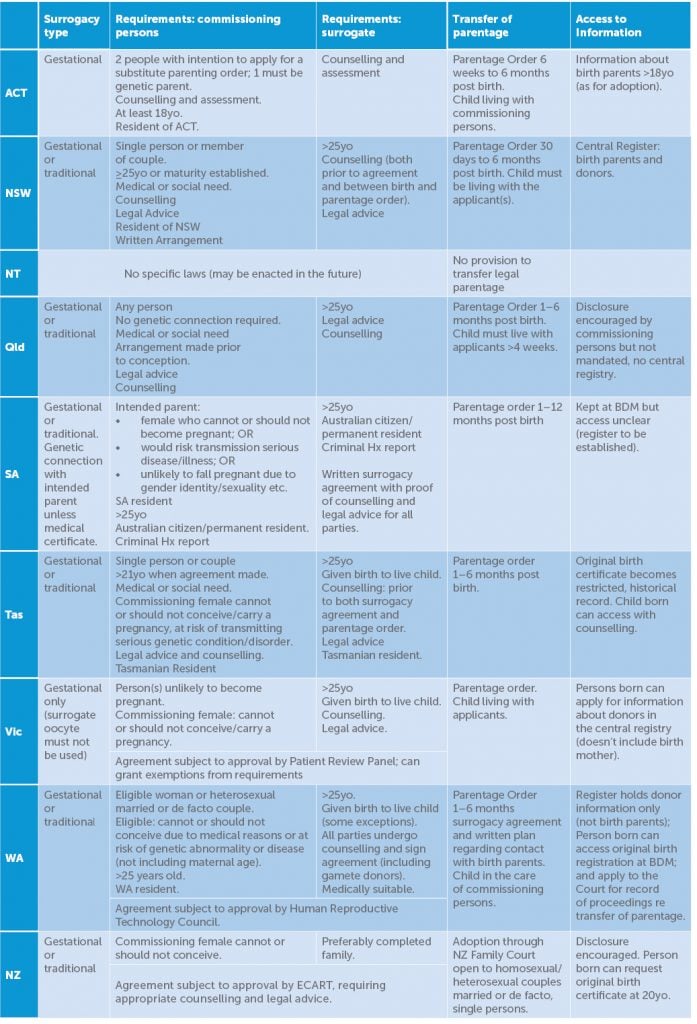
Jurisdictions vary regarding whether a surrogate may use her own ova (‘traditional’) or not (‘gestational’), the requirements of commissioning persons and surrogate, and method of transfer of legal parentage (Table 1).
International surrogacy
It is an offence for residents of the ACT, NSW and Queensland to travel to another country to engage in commercial surrogacy. When people do engage in international commercial surrogacy from Australia, a child born as a result may be granted ‘Australian citizenship by descent’ if one or both intended parents are its genetic parent(s). Thereafter, Family Court granted ‘parenting’ orders may be sought and obtained on a case-by-case basis; however, State Court ‘parentage’ orders will not be granted in cases of identified commercial surrogacy. In NZ, in the absence of citizenship by descent, the Minister of Immigration may consider the temporary entry of a child born as the result of an international surrogacy arrangement on a case-by-case basis. A genetic link is required between at least one of the commissioning parents and the child, as well as evidence that adoption proceedings are underway with the New Zealand Family Court.
Table 1. Surrogacy requirements across jurisdictions.
References
- NHMRC. The Ethical guidelines on the use of assisted reproductive technology in clinical practice and research (ART guidelines). 2017. Available from: www.nhmrc.gov.au/about-us/publications/ethical-guidelines-use-assisted-reproductive-technology.
- NHMRC. The Ethical guidelines on the use of assisted reproductive technology in clinical practice and research (ART guidelines). 2017. Available from: www.nhmrc.gov.au/about-us/publications/ethical-guidelines-use-assisted-reproductive-technology.






Leave a Reply