The rise of antimicrobial resistance is leading to increased maternal and neonatal morbidity and mortality.1 Antimicrobials pose a self-defeating conundrum; every dose used contributes to increased resistance and loss of efficacy. Unnecessary antimicrobial prescribing and preventable infections or colonisation with multiresistant organisms (MROs) compound the problem. In recognition of this, antimicrobial stewardship strategies are now mandated in the National Safety and Quality Health Service standards for health service accreditation.2 In this article, we discuss key strategies of an antimicrobial stewardship program, as well as the role of infection prevention and control (IPC), antibiotic allergy delabelling, and antimicrobial prophylaxis in minimising antimicrobial resistance and harms to mother and neonate. There is also an urgent need for international collaboration between industry, research, government and non-government organisations, to find new ways to support research and development of novel antimicrobials.
Box 1. Six principles of optimal antimicrobial use.
- right patient (with [or at risk of] an infection for which antimicrobial therapy [or prophylaxis], is indicated)
- right time (eg. as soon as possible after onset of sepsis or at appropriate time for prophylaxis [Table 2])
- right drug choice (with as narrow a spectrum as possible for the likely or known pathogen)
- right route (orally, as soon as clinically feasible)
- right dose (to achieve therapeutic level at the site of infection)
- right duration (no longer than necessary to achieve control of, or prevent, infection)
- Antimicrobial stewardship
Antimicrobial stewardship promotes optimal antimicrobial prescribing (Box 1) while minimising the adverse consequences related to antimicrobial toxicity and resistance, and unnecessary costs. The implementation of an effective antimicrobial stewardship program (Table 1) requires the coordinated efforts of infectious diseases specialists, pharmacists, IPC professionals, microbiologists and hospital or practice administrators to improve and measure the appropriate use of antibiotics (Box 2).
Table 1. Key components of an antimicrobial stewardship program.3
| Inpatient setting | Outpatient setting |
|
|
Box 2. Benefits of an antimicrobial stewardship program.
- Improvement in patient outcomes (eg. fewer adverse drug effects, decreased length of hospitalisation, fewer readmissions)
- Optimal (more effective) antimicrobial use
- Reduction in inappropriate antimicrobial use
- Reduction in rates of resistant organisms
- Reduction in rates of C. difficile infection
- Adherence to institutional pathways and protocols
- Reduction in antimicrobial associated healthcare costs
Importance of infection prevention and control
In the 1840s, Ignaz Semmelweis changed modern medicine when he showed that hand hygiene in an Austrian obstetric clinic reduced mortality from more than 10 per cent to 2 per cent. This lesson rings ever true, especially in the face of increasing antimicrobial resistance (AMR). There are high levels of morbidity from multiresistant organisms (MROs) such as methicillin-resistant Staphylococcus aureus (MRSA), S. aureus with reduced vancomycin susceptibility (hVISA and others), multi-resistant Gram-negative bacilli and vancomycin resistant enterococci. Routine screening for MROs in obstetric patients is not currently recommended,4 as most colonised mothers do not develop invasive disease. However, undetected MRO colonisation in mothers risks the unintentional introduction of MROs into neonatal units. Awareness of risk factors for colonisation (Box 3) and their detection remain important in the management of symptomatic maternal and neonatal infection. Regardless, strict IPC programs are instrumental in limiting their nosocomial spread. Key elements of IPC programs include measures to reduce inappropriate antibiotic exposure, aseptic technique for invasive procedures, appropriate use of personal protective equipment, screening for MROs when indicated and contact precautions for colonised or infected patients.5
Box 3. Major risk factors for multidrug resistant organism colonisation.
- Prolonged hospital admission (with or without antibiotic therapy)
- Admission to intensive care unit
- Recent, especially healthcare-related, travel to a high AMR-prevalence country
- Previous history of MRO colonisation
- Transfer from long-term care facility
- Chronic wounds or indwelling medical device
- Previous prolonged or repeated antibiotic therapy
Antibiotic allergy delabelling
Antimicrobial stewardship programs provide guidance on antibiotic allergy delabelling and correct second line antibiotic choice when a true allergy exists. False labelling of adverse drug reactions as allergies leads to inappropriate use of second line antibiotics and increased antimicrobial resistance. Correct allergy labelling is further complicated by the fact that up to 80 per cent of patients with true IgE mediated allergic reactions lose these responses over time.6
Careful prenatal history-taking can help delineate non-immune-mediated Type A from immune-mediated Type B, drug reaction;7 (Figure 1). A common clinical scenario is the choice of antibiotic in a pregnant woman colonised with Streptococcus agalactiae (Group B Streptococcus or GBS), who has a history of penicillin reactions. Women with Type A reactions have low risk of anaphylaxis; first-generation cephalosporins remain the preferred choice. Clindamycin is recommended for women with a high penicillin anaphylaxis risk, if the isolate is known to be susceptible. To facilitate this, sensitivity testing should be requested specifically at the time of GBS culture. Otherwise, vancomycin is now the preferred option in severe penicillin allergy due to rising clindamycin resistance rates globally.8 9 Australian data showed 4.2 per cent clindamycin resistance in GBS in 201110 and, more recently, rates as high as 20 per cent have been reported.11
Drug exanthems differ from urticaria by being non-pruritic and often morbilliform, rather than pruritic with a ‘wheal and flare’ phenomenon. It is usually safe to rechallenge patients who have had drug exanthems, while there is a risk of anaphylaxis with rechallenge of IgE-mediated reactions. In difficult histories, skin testing can be used to determine the likelihood of IgE mediated reactions. It should never be used to evaluate patients with severe non-IgE mediated reactions such as Stevens-Johnson syndrome or toxic epidermal necrolysis, as it cannot predict the recurrence of these reactions.
Ideally, all patients with a history of immune-mediated allergy should be referred to an antibiotic allergy delabelling service for specialist assessment.
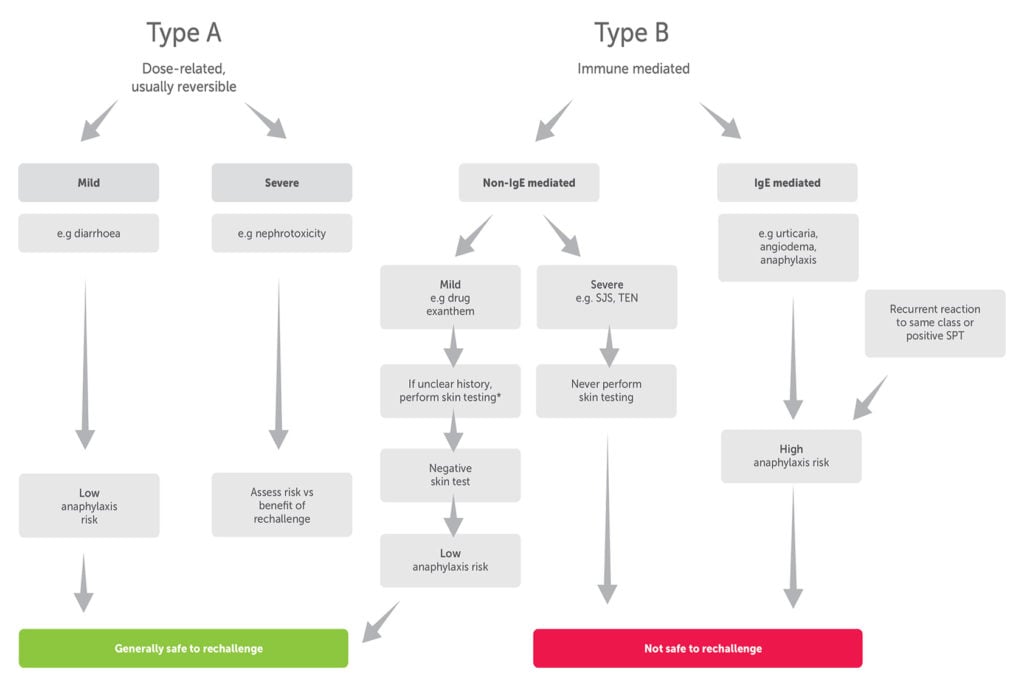
Figure 1. Drug reaction types and implications for drug allergy delabelling. (Adapted from Practical Implementation of an Antibiotic Stewardship Program).
Antimicrobial prophylaxis
Correct antimicrobial prophylaxis can prevent infection and reduce morbidity and mortality; however, inappropriate peripartum antimicrobial prophylaxis risks increased antimicrobial resistance and infant gut microbiota dysbiosis.12 Antimicrobial prophylaxis in the peripartum period targets polymicrobial flora in the genital tract to prevent intra-amniotic infection, postpartum endometritis or surgical site infection, in women at risk. The major ascending cervicovaginal pathogens include group A beta-haemolytic streptococcus (Streptococcus pyogenes), GBS, S. aureus including MRSA, anaerobic cocci and enteric Gram-negative bacilli.13 14 15 Genital mycoplasmas are commonly isolated from patients with intra-amniotic infection, but the clinical significance of these organisms is uncertain.16
The World Health Organization (WHO) released guidance on prevention and treatment of peripartum17 and surgical site infections,18 in 2015 and 2016 respectively. Antibiotic prophylaxis is not recommended for women with prelabour rupture of membranes at more than 36 weeks gestation, for women with meconium-stained amniotic fluid, uncomplicated vaginal births or vacuum- or forceps-assisted operative vaginal births. Antibiotic prophylaxis is recommended for women undergoing manual removal of the placenta and for third- and fourth-degree perineal tears, but not for episiotomy.
Table 2. Key recommendations relating to surgical prophylaxis in obstetrics and gynaecology, adapted from eTG19 and WHO guidelines 2016.20
| Timing of administration of surgical prophylaxis | Short-acting antibiotics, such as cefazolin, < 60 min prior to incision. Long-acting antibiotics, < 120 min prior to incision. Vancomycin requires a slow infusion; started at least 15 min prior to incision; surgical incision can occur before the infusion is completed. |
| Prophylaxis duration | Single preoperative dose +/- intraoperative redosing usually adequate in caesarean and gynaecologic surgery. Antimicrobial prophylaxis should not be continued because of the presence of a wound drain for the purpose of preventing surgical site infections. |
| Intraoperative redosing | Significant delay in starting the operation More than two half-lives of the drug have elapsed since the previous dose (eg. 4 hours for cefazolin) > 1.5 L blood lost during adult procedure |
Caesarean section is the most important risk factor for maternal infection in the immediate postpartum period, with aseptic surgical techniques and use of prophylactic antibiotics being the cornerstones of infection prevention. WHO recommends vaginal cleansing with povidone-iodine immediately before caesarean section to reduce postpartum endometritis. See Table 2 for key recommendations regarding timing, duration (including in the presence of drains) and intraoperative redosing in preventing surgical site infections. Please refer to Therapeutic Guidelines (eTG)21 for the updated guidelines for prophylaxis and empirical therapy in intrapartum and postpartum infections.
Summary
The world is running out of useful antibiotics in the face of rising antimicrobial resistance. With the increasing implementation of antimicrobial stewardship, along with the principles of antibiotic allergy delabelling, strict IPC and antimicrobial prophylaxis, we aim to empower colleagues to responsibly prescribe antimicrobials, not just for the safety of mothers and neonates, but for our global community.
References
- Limmathurotsakul D, Dunachie S, Fukuda K, et al. Improving the estimation of the global burden of antimicrobial resistant infections. Lancet Infect Dis. 2019;19(11):e392-398.
- Australian Commission on Safety and Quality in Health Care. Standard 3 Preventing and Controlling Healthcare Associated Infections: Safety and Quality Improvement Guide. 2012. Available from: www.safetyandquality.gov.au/sites/default/files/migrated/Standard3_Oct_2012_WEB.pdf.
- eTG complete. Melbourne: Therapeutic Guidelines Limited; 2019. Available from: www.tg.org.au.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 199: Use of Prophylactic Antibiotics in Labor and Delivery. Obst Gynecol. 2018;132(3):e103-19.
- Australian Commission on Safety and Quality in Health Care. Multi-resistant organism screening and clearance recommendations. Healthcare Infection Surveillance Subcommittee of the Healthcare Associated Infections Advisory Committee, National Quality and Safety Council; 2004.
- Barlam TF, Neuhauser MM, Tamma PD, et al. Practical Implementation of an Antibiotic Stewardship Program. Cambridge: Cambridge University Press; 2018.
- eTG complete. Melbourne: Therapeutic Guidelines Limited; 2019. Available from: www.tg.org.au.
- Hughes RG, Brocklehurst P, Steer PJ, et al. Prevention of Early-onset Neonatal Group B Streptococcal Disease. Green-top Guideline No. 36. BJOG. 2017;124(12):e280-305.
- Prevention of Group B Streptococcal Early-Onset Disease in Newborns: ACOG Committee Opinion, Number 782. Obstet Gynecol. 2019;134(1):e19-40.
- Garland SM, Cottrill E, Markowski L, et al. Antimicrobial resistance in group B streptococcus: the Australian experience. J Med Microbiol. 2011;60(Pt 2):230-5.
- RANZCOG. Maternal Group B Streptococcus in pregnancy: Screening and management (C-Obs 19). 2019. Available from: ranzcog.edu.au/statements-guidelines/Obstetrics/Maternal-Group-B-Streptococcus-(GBS)-in-Pregnancy.
- Azad MB, Konya T, Persaud RR, et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG. 2016;123(6):983-93.
- Czikk MJ, McCarthy FP, Murphy KE. Chorioamnionitis: from pathogenesis to treatment. Clinical Microbiology and Infection. 2011;17(9):1304-11.
- Bacterial Sepsis following Pregnancy. Green-top Guideline No. 64b. Royal College of Obstetricians and Gynaecologists; 2012.
- Palasanthiran P, Starr M, Jones C, Giles M, editors. Management of Perinatal Infections. 2014 ed: Australasian Society for Infectious Diseases (ASID) Inc; 2014.
- eTG complete. Melbourne: Therapeutic Guidelines Limited; 2019. Available from: www.tg.org.au.
- WHO Recommendations for Prevention and Treatment of Maternal Peripartum Infections. WHO Guidelines Review Committee. Geneva: World Health Organisation; 2015.
- Allegranzi B, Zayed B, Bischoff P, et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis. 2016;16(12):e288-303.
- eTG complete. Melbourne: Therapeutic Guidelines Limited; 2019. Available from: www.tg.org.au.
- Allegranzi B, Zayed B, Bischoff P, et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis. 2016;16(12):e288-303.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 199: Use of Prophylactic Antibiotics in Labor and Delivery. Obst Gynecol. 2018;132(3):e103-19.





Leave a Reply