Dr Haruo Usuda is an academic-neonatologist and currently a Visiting Research Fellow at the University of Western Australia, where he leads the artificial placenta development program. A/Prof Matt Kemp heads the WIRF Perinatal Laboratories at the University of Western Australia. His current research program focuses on the development of novel interventions for preterm infants and the optimisation of antenatal steroid therapy. The authors would like to gratefully acknowledge the support of the Women and Infants Research Foundation; the Channel 7 Telethon Trust; the Department of Health, Government of Western Australia; and the National Health and Medical Research Council for supporting their research.
The need for a new life support system
Preterm birth remains the leading cause of neonatal death in Australia today, with some 8 per cent of all Australian babies being delivered prior to 37 weeks completed gestation.1 Worldwide, an estimated 15 million preterm babies are born every year and, of these, around one million will die from complications of prematurity.2 Rates of preterm birth are highest in socio-economically disadvantaged groups, such that highest rates of preterm deliveries are seen in regions of the United States, Africa and South-East Asia.3 Here in Australia, the rate of preterm birth in Aboriginal and Torres Strait Islander populations is nearly double that of the non-Indigenous population.
Even in high-resource environments such as Australia, with socialised medicine providing ready access to excellent obstetric and neonatal care, being born too early conveys an increased absolute risk of developing a range of diseases affecting the respiratory, cardiovascular and sensory systems; as might be expected, the earlier one is born, the greater the absolute risk and severity of complications.4 Due to profound advances in obstetric care and perinatal medicine, outcomes for almost all preterm babies have improved markedly over the past 50 years. Although the majority of improvements in outcomes likely derive from advances in pregnancy and neonatal care in toto, the introduction of antenatal steroid therapy and exogenous surfactant, combined with the availability of increasingly sophisticated ventilation equipment have profoundly improved the chances of preterm babies being discharged home free of a major morbidity.
The one exception to this preterm birth success story is the small number of babies born extremely early at the border of viability – between 21 and 24 weeks completed gestation – a resistant rump of babies at high risk of death or the development of lifelong disease. In this particular demographic, the rates of death, or survival to discharge with a major morbidity, remain stubbornly high.5 Data presented in 2015 by Stoll and colleagues in the Journal of the American Medical Association serve to demonstrate the challenges facing practitioners working with extremely preterm infants, some weighing as little as 400 g. Stoll’s data show that over the period 1993–2012, approximately two-thirds of infants born at 23 weeks gestation died of complications associated with prematurity.6 At less than 24 weeks’ gestation, fewer than one in five babies survived to discharge without a major morbidity. There were only small improvements in mortality rates for these extremely preterm infants, and no improvements in rates of disease-free discharge for babies born at or below 24 weeks gestation.7 In contrast, survival to discharge among all infants born at 28 weeks gestation – just one month later – was reported as 94 per cent, and disease-free survival to discharge as 56 per cent – a profound improvement.8
We believe that within the explanation for this marked difference in survival also lies the answer to improving outcomes for the babies born at the border of viability. Current neonatal therapies are, broadly speaking, based around two fundamental assumptions: i) that the preterm lung may be used for gas exchange and; ii) that given the initiation of pulmonary gas exchange, the preterm cardiovascular system must precociously adapt to life outside the uterus, closing the fetal shunts. As will be discussed in greater detail below, a number of investigators have proposed that the best way to save the lives of these extremely preterm babies is for them not to breathe at all, and to leave the fetal shunts patent as they normally would be in utero.
A physiological justification for such an approach is provided by a simple ontological assessment of fetal lung development. The canalicular phase of lung development occurs in humans over approximately an eight-week period between 16 and 24 weeks gestation. Respiratory bronchioles develop during this phase, which precedes formation of both alveolar ducts and sacs, and the production of pulmonary surfactant. As such (and ignoring a host of other developmental modifications occurring in the lung over this period) the surface area available for gas exchange is comparatively small and the compliance of the lung is comparatively low. Taken together, the efficiency of gas exchange is poor and requires additional and potentially injurious inspiratory pressure to obtain an appropriate tidal volume. Put another way, the preterm lung at 21–24 weeks gestation is poorly suited to performing gas exchange; a reality reflected in the poor outcomes seen among today’s extremely preterm infants.
Acknowledging that current neonatal life-support technologies based on pulmonary ventilation have met an efficacy threshold when applied to patients below 24 weeks gestation, we have sought to extend the work of a number of investigators in Japan, Korea, Canada and the United States to develop a non-pulmonary life support platform for extremely preterm infants born at the border of viability. A key element of our approach in this domain is a change from treating these infants as small babies, to treating them as fetuses and working with, rather than against, their current developmental state.
What is an artificial placenta?
The human placenta performs a myriad number of essential functions to support fetal growth. In contrast, artificial placentas that have been developed for use in the treatment of extremely preterm infants are largely limited in function to performing gas exchange. Thus the artificial placenta may better be characterised as an extra-corporeal membranous oxygenation (ECMO) system. In our own centre,9 this function is supplemented with intravenous administration of a cocktail of medicines, nutrients and fluids necessary to maintain key physiological variables within their respective reference ranges, and to maintain ductal patency, all contained within an artificial uterus filled with a sterile artificial amniotic fluid (Figure 1). As might be expected, there are a range of circuit configurations, with the most promising designs to date incorporating an arterio-venous circuit secured to the umbilical vasculature and driven by the fetal heart.
A (very) brief history of the artificial placenta
At first pass, the concept of maintaining an extremely preterm fetus on an artificial placenta, safely submerged in an artificial uterus replete with synthetic amniotic fluid and in the absence of pulmonary gas exchange, sounds like a futuristic proposition. As it happens, efforts to develop an artificial placenta to support extremely preterm infants have been a work in progress for over 60 years – although the target demographic today is almost certainly different (much smaller and more immature) than that envisaged prior to the adoption of antenatal steroids and exogenous surfactant. Among the first reported use of an artificial placenta to support pre-viable human fetuses is contained in a paper by Westin and colleagues published in Acta Pediatrica in 1958 – the same year that Qantas launched its round-the-world express service from Melbourne (via Nadi; Honolulu; San Francisco; New York; London; Rome; Athens; Karachi; New Delhi; Bangkok; Singapore; Jakarta; and Perth). Of work from groups in Canada,10 Japan11 and Korea,12 perhaps the most important report was a landmark study by Unno and colleagues in 1993,13 which reported the long-term survival of preterm goats treated for 21 days on an artificial placenta and a subsequent 30 days on mechanical ventilation. Interestingly, the authors noted that preterm goats failed to spontaneously breathe after the withdrawal of ventilation support, potentially due to the extensive use of the neuromuscular inhibitor pancuronium bromide throughout the experiment. More recently, groups from the United States14 15 have used preterm sheep to perform exciting pre-clinical studies demonstrating the potential clinical utility of the artificial placenta as a life-support platform for extremely preterm infants.
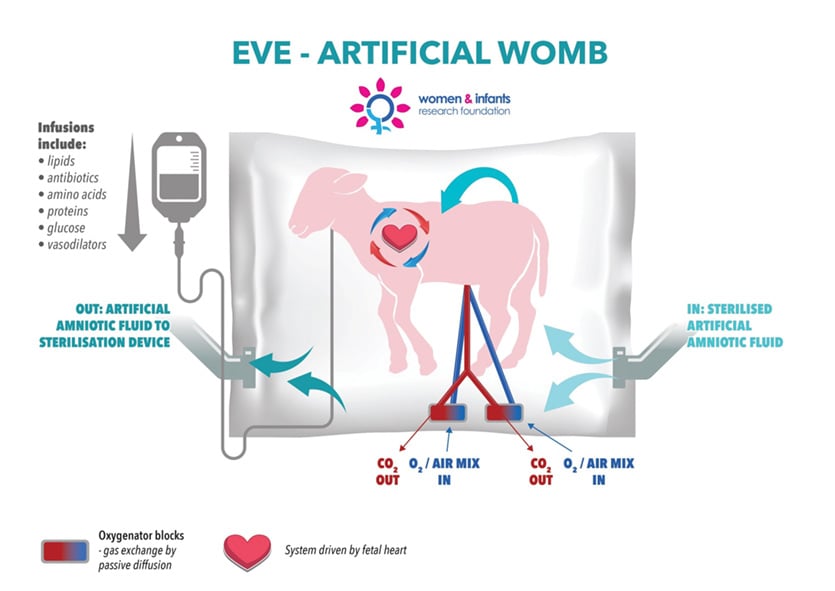
Figure 1. The workings of the artificial womb.
Bilateral Japanese-Australian development of the artificial placenta
In 2013, investigators in Perth (Profs John Newnham and Matt Kemp) and Sendai, Japan (Prof Masatoshi Saito, Dr Haruo Usuda, Dr Yuichiro Miura, Dr Shimpei Watanabe, Dr Takushi Hanita, Dr Shinichi Sato), with the support of Nipro Corporation (based in Osaka, Nipro Corporation is one of Japan’s leading medical technology companies) and the Women and Infants Research Foundation (www.wirf.com.au; one of Australia’s peak not-for-profits focused on preterm birth prevention and improved preterm outcomes) formed a bilateral consortium to develop a new life-support platform for extremely preterm infants, based on the use of an artificial placenta developed in preliminary studies at Tohoku University in Sendai, Japan. The collaboration draws on respective surgical, engineering and medical research strengths from each partner institution; the platform has also taken on something of an Australian identity, with members of the team taking inspiration from the unique reproductive cycle of marsupials, and naming it a ‘Joey’. Since the consortium’s establishment, and with support from the Channel 7 Telethon Trust, the Department of Health (WA) and the National Health and Medical Research Council, the team has undertaken an intensive series of experiments with preterm lambs to develop a high-efficiency, arterio-venous gas exchange system driven solely by the fetal heart (Figure 1). In 2014, we achieved variable survival for 48 hours on a prototype system; in 2015 we achieved stable maintenance of key physiological parameters for 48 hours.16 In 2016, we achieved optimal maintenance of physiological parameters in preterm lambs for one week and successfully transferred a preterm lamb onto pulmonary ventilation.17 Ultrasound and necroscopy data revealed a normal pattern of growth. All haematological parameters were normal, and all lambs were infection-free. In 2017, we extended this healthy survival period to two weeks. In 2018, in experiments currently under publication embargo, we were successful, for the first time, in adapting extremely preterm lambs to our next-generation artificial placenta system and maintaining them for an extended period of time.
Coming soon to a nursery near you?
There can be no doubt that the artificial placenta field, after a lengthy and iterative development process, is rapidly accelerating towards clinical application. However, unless there is a very significant body of experimental data that is not yet in the public domain, our assessment is that it will be many years of painstaking research before a life-support platform based on an artificial placenta is appropriate for clinical application. Noting that we would be happy to be proven wrong (such is the need for this technology) our view on this point remains that the experimental studies presented to date neither sufficiently inform nor justify the risk of current clinical application.
An assessment of the latest data from the field (including our own), against a likely clinical presentation for a putative ‘first artificial placenta patient’ serves to highlight the challenges yet to overcome, noting of course that any artificial placenta-based therapy must be demonstrably better (that is, better chance of disease-free survival) than any treatment it may replace. For example, against data presented by Stoll and colleagues,18 this equates to at least a better than 50 per cent survival rate for a 23-week preterm infant. We presently have no short- or long-term outcome data for extremely preterm sheep or goats maintained on an artificial placenta platform – with published data in our possession being from moderately preterm sheep or goats (105–120 days gestation out of 150 days, and all >1000 g in delivery weight). We have no data from non-human primates. The data presented to date, of which we are aware, derives from fetuses delivered in an optimal state of health from a pregnancy that would have otherwise continued. Accordingly, investigators have not had to contend with the added complication of managing an extremely preterm fetus with limited cardiovascular compensation capacity and a host of other developmental challenges (such as adrenal insufficiency) in the setting of growth restriction, chorioamnionitis/funisitis, or systemic fetal infection – all common aetiological factors of extremely early preterm birth.
Sitting alongside these pragmatic considerations is the need for the scientific, medical, legal and wider community to embark on a discussion of the ethics of an artificial placenta – preferably well in advance of such a platform entering clinical practice. The use of an artificial placenta to maintain otherwise ‘non-viable’ fetuses raises a host of legal and ethical queries: when would such a baby be considered to have been ‘born’ and when would it obtain legal status as a person? A clearly articulated consideration of these issues, based on broad consultation, will form a critically important part of the journey of artificial placenta technology to the clinic.
For our own contribution, we eagerly anticipate working with our colleagues in Japan and Australia to drive this highly promising technology towards clinical application.
References
- Newnham JP, Kemp MW, White SW, et al. Applying precision public health to prevent preterm birth. Frontiers in Public Health. 2017;5:66.
- Blencowe H, Cousens S, Chou D, et al. Born Too Soon: The global epidemiology of 15 million preterm births. Reproductive Health. 2013;10:S2.
- Blencowe H, Cousens S, Chou D, et al. Born Too Soon: The global epidemiology of 15 million preterm births. Reproductive Health. 2013;10:S2.
- Romero R, Dey SK, Fisher SJ. Preterm labor: One syndrome, many causes. Science. 2014;345:760-5.
- Stoll BJ, Hansen NI, Bell EF, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993-2012. JAMA. 2015;314:1039-51.
- Stoll BJ, Hansen NI, Bell EF, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993-2012. JAMA. 2015;314:1039-51.
- Stoll BJ, Hansen NI, Bell EF, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993-2012. JAMA. 2015;314:1039-51.
- Stoll BJ, Hansen NI, Bell EF, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993-2012. JAMA. 2015;314:1039-51.
- Usuda H, Watanabe S, Miura Y, et al. Successful maintenance of key physiological parameters in preterm lambs treated with ex vivo uterine environment therapy for a period of 1 week. Am J Obst Gynecol. 2017;217:457.e1-57. e13.
- Callaghan JC, Angeles J, Boracchia B, et al. Studies of the first successful delivery of an unborn lamb after 40 minutes in the artificial placenta. Can J Surg. 1963;6:199-206.
- Kuwabara Y, Okai T, Kozuma S, et al. Artificial Placenta: Long-term extrauterine incubation of isolated goat fetuses. Artificial Organs. 1989;13:527-31.
- Pak SC, Song CH, So GY, et al. Extrauterine Incubation of Fetal Goats Applying the Extracorporeal Membrane Oxygenation via Umbilical Artery and Vein. J Korean Med Sci. 2002;17:663-8.
- Unno N, Kuwabara Y, Okai T, et al. Development of an Artificial Placenta: Survival of Isolated Goat Fetuses for Three Weeks with Umbilical Arteriovenous Extracorporeal Membrane Oxygenation. Artificial Organs. 1993;17:996-1003.
- Partridge EA, Davey MG, Hornick MA, et al. An extra-uterine system to physiologically support the extreme premature lamb. Nat Commun. 2017;8:15112.
- Bryner B, Gray B, Perkins E, et al. An extracorporeal artificial placenta supports extremely premature lambs for 1 week. J Pediatr Surg. 2015;50:44-9.
- Miura Y, Saito M, Usuda H, et al. Ex-Vivo Uterine Environment (EVE) Therapy Induced Limited Fetal Inflammation in a Premature Lamb Model. PLoS One. 2015;10:e0140701.
- Usuda H, Watanabe S, Miura Y, et al. Successful maintenance of key physiological parameters in preterm lambs treated with ex vivo uterine environment therapy for a period of 1 week. Am J Obst Gynecol. 2017;217:457.e1-57. e13.
- Stoll BJ, Hansen NI, Bell EF, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993-2012. JAMA. 2015;314:1039-51.





Leaving premies with their own placenta will greatly improve their outcomes. The loss of the placenta involves an amputation of the child’s organ. My 30 years experience with lotus birth leaving the cord intact to come away naturally at the navel has me totally convinced that it is of substantial benefit for the child. Firstly it ensures the baby receives its full placental transfusion of 30-50% of total blood volume at birth. Deprivation of this essential and most valuable resource creates extreme stress for the newborn. We have measured the Cranial Rhythmic Pulse (CRI) which has a slower rhythm than the arterial respiratory rhythm with a frequency around 8-12 oscillations per minute and is found in all tissues of the living body in the placenta in the days after birth. The placenta continued to pulse in unison with the baby’s CRI until it came away at the navel naturally three days after birth. There is much to learn about this highly intelligent organ as you are finding out. Before making an ‘artificial one is it not wise to investigate what the real one has to offer?