Diabetes in pregnancy is associated with a range of neonatal complications, with infants of diabetic mothers at increased risk for mortality and morbidity, compared to those born to non-diabetic mothers. The management of the infant requires anticipation, early recognition and treatment of potential complications, while also providing routine neonatal care.
Fetal effects
The development and severity of complications in the fetus of a diabetic mother is influenced by the degree, timing and duration of maternal hyperglycaemia. Good maternal glycaemic control during pregnancy reduces the risk of some, but not all, of the potential complications in infants. Diabetic embryopathy can occur if maternal hyperglycaemia is present at the time of conception and in the first trimester (generally with pre-existing type 1 or type 2 diabetes). This can result in birth defects and spontaneous abortions.1 In the second and third trimesters, maternal hyperglycaemia results in fetal hyperglycaemia and subsequent fetal hyperinsulinaemia, leading to a range of complications in the neonate.2
Neonatal effects
Complications can be present at birth or develop later in the neonatal period. They can be categorised into congenital abnormalities or birth/neonatal complications (Table 1).3
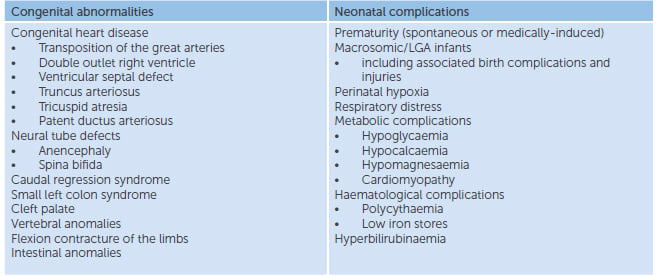
Table 1. Potential neonatal complications in the infant of a diabetic mother.
The physical examination of the infant should occur soon after birth, identifying any potential congenital anomalies, assessing respiratory and cardiac status, and assessing for the presence or absence of macrosomia, metabolic and haematological complications.
The infants have increased rates of neonatal intensive care unit admissions, compared to those born to non-diabetic mothers, however, unless there is clinical need for admission to the nursery (for example, blood glucose monitoring), infants should ideally remain on the postnatal ward for routine surveillance and monitoring.
Macrosomia
Macrosomia, defined as a birth weight above the 90th percentile or greater than 4000g, is associated with disproportionate growth, with a subsequent predisposition to birth injuries, such as shoulder dystocia, clavicle fractures, brachial plexus injuries and perinatal asphyxia.4 5 Macrosomic neonates are at increased risk for respiratory distress and metabolic complications.6 7 The early identification of a macrosomic infant (ideally prenatally) is essential in allowing for a timely and safe delivery, with prevention, minimisation or early detection of complications.
Respiratory distress
Respiratory distress is common and can be multifactorial in nature.8 9 Maternal hyperglycaemia appears to delay surfactant synthesis, resulting in an increased risk of respiratory distress syndrome, affecting both preterm and term neonates. Infants also have increased rates of transient tachypnoea of the newborn, cardiomyopathy and are more likely to be born prematurely,10 thereby increasing the risk of respiratory issues in the neonatal period. Targeted management of respiratory distress should focus on early identification of the underlying cause.
Metabolic complications
Hypoglycaemia
Fetal hyperglycaemia results in hyperinsulinaemia, through hypertrophy and hyperfunctioning of the beta cells in the fetal pancreas.11 After birth, maternally-derived hyperglycaemia resolves. However, there is transient continuation of the hyperinsulinaemic state, as neonatal beta cells adjust to normal neonatal blood glucose levels. This hyperinsulinaemic state typically normalises within two to four days of birth.
While definitions of hypoglycaemia are somewhat arbitrary and still debated, in Australia, neonatal hypoglycaemia is commonly defined as a blood glucose of less than 2.6mmol/L.12 Symptoms of hypoglycaemia are broad and may include poor feeding, jitteriness, irritability, apnoea, cyanosis, hypotonia, lethargy, seizures, pallor, sweating, tachycardia, vasomotor instability, bradycardia and hypotension.[/note] 13 Most infants with low blood glucose levels are initially asymptomatic and detection is based on routine surveillance. Infants of diabetic mothers with poor recent control, on high doses of insulin, or those with type 1 diabetes, are considered at high risk for hypoglycaemia and are often admitted directly to the nursery. All infants of diabetic mothers require early establishment of feeds and regular monitoring of blood glucose levels.
The management of hypoglycaemia depends on the timing and severity. Generally, for mild to moderate hypoglycaemia, management with oral or enteral feeds is appropriate and preferred. For severe hypoglycaemia (or in those infants with other complications such as prematurity or respiratory distress), intravenous management is required. Glucose gel (40 per cent) is now readily available and can be used for mild to moderate hypoglycaemia in infants whose glucose levels do not respond to an enteral feed, or in those with severe hypoglycaemia, where a delay in IV access is unavoidable. Blood glucose screening can generally cease after three consecutive readings of 2.6mmol/L or greater, if the infant is feeding well.14
Hypocalcaemia and hypomagnesaemia
Hypocalcaemia and hypomagnesaemia are usually transient, asymptomatic and resolve without treatment.15 However, they should be considered if the neonate is symptomatic (for example, seizures, jitteriness), or otherwise unwell. Treatment is through oral or intravenous supplementation.
Cardiomyopathy
Fetal hyperinsulinaemia can lead to cardiac hypertrophy through increased synthesis and deposition of fat and glycogen in the myocardial cells.16 Most neonates are asymptomatic, however, 5–10 per cent develop respiratory distress, signs of poor cardiac output or cardiac failure. Chest x-ray may show cardiomegaly and echocardiograph is diagnostic. Cardiomyopathy is transient, resolving with normalisation of plasma insulin levels,17 therefore, management is supportive (intravenous fluids, propranolol).
Haematological complications
Polycythaemia and low iron stores
Polycythaemia, defined as a haematocrit of greater than 0.65 per cent, is thought to be a result of chronic fetal hypoxaemia and oxidative stress, leading to increased erythropoietin concentrations. Iron is believed to shunt into the red cell mass, resulting in low iron stores. The haematocrit should be measured within 12 hours of birth. Most polycythaemic infants are asymptomatic and can be managed supportively with careful monitoring of hydration status and glucose levels. Symptomatic infants, or those with severe polycythaemia, may require intravenous hydration or partial exchange transfusions.18 Iron supplementation is not recommended, as iron reutilisation occurs after the breakdown of the red cell mass.
Long-term effects
While most complications in infants of diabetic mothers are managed successfully in the neonatal period, there is evidence that prenatal exposure to hyperglycaemia can result in long-term metabolic and possibly neurodevelopmental complications. Ongoing follow up is required.
Conclusion
Infants of diabetic mothers are known to be at increased risk for a variety of complications in the neonatal period, with increased mortality and morbidity, compared to infants born to non-diabetic mothers. Management should focus on good prenatal care and early identification and treatment of complications.
References
- Buchanan TA, Kitzmiller JL. Metabolic interactions of diabetes and pregnancy. Annu Rev Med 1994; 45:245.
- Buchanan TA, Kitzmiller JL. Metabolic interactions of diabetes and pregnancy. Annu Rev Med 1994; 45:245.
- Cordero L, Treuer SH, Landon MB, Gabbe SG. Management of infants of diabetic mothers. Arch Pediatr Adolesc Med 1998; 152:249.
- Persson M, Fadl H, Hanson U, Pasupathy D. Disproportionate body composition and neonatal outcome in offspring of mothers with and without gestational diabetes mellitus. Diabetes Care 2013; 36:3543.
- Esakoff TF, Cheng YW, Sparks TN, Caughey AB. The association between birthweight 4000g or greater and perinatal outcomes in patients with and without gestational diabetes mellitus. Am J Obstet Gynecol 2009; 200:672.e1.
- Persson M, Fadl H, Hanson U, Pasupathy D. Disproportionate body composition and neonatal outcome in offspring of mothers with and without gestational diabetes mellitus. Diabetes Care 2013; 36:3543.
- Esakoff TF, Cheng YW, Sparks TN, Caughey AB. The association between birthweight 4000g or greater and perinatal outcomes in patients with and without gestational diabetes mellitus. Am J Obstet Gynecol 2009; 200:672.e1.
- Cordero L, Treuer SH, Landon MB, Gabbe SG. Management of infants of diabetic mothers. Arch Pediatr Adolesc Med 1998; 152:249.
- Bourbon JR, Farrell PM. Fetal lung development in the diabetic pregnancy. Pediatr Res 1985; 19:253.
- Cordero L, Treuer SH, Landon MB, Gabbe SG. Management of infants of diabetic mothers. Arch Pediatr Adolesc Med 1998; 152:249.
- Buchanan TA, Kitzmiller JL. Metabolic interactions of diabetes and pregnancy. Annu Rev Med 1994; 45:245
- Royal Prince Alfred Hospital Newborn Guidelines – Hypoglycaemia: www.slhd.nsw.gov.au/rpa/neonatal/protocols.html
- Committee on Fetus and Newborn. Adamkin DH. Postnatal glucose homeostasis in late-preterm and term infants. Pediatrics 2011; 127:575.
- Royal Prince Alfred Hospital Newborn Guidelines – Hypoglycaemia: www.slhd.nsw.gov.au/rpa/neonatal/protocols.html.
- Cordero L, Treuer SH, Landon MB, Gabbe SG. Management of infants of diabetic mothers. Arch Pediatr Adolesc Med 1998; 152:249.
- Tyrala EE. The infant of the diabetic mother. Obstet Gynecol Clin North Am 1996; 23:221.
- Way GL, Wolfe RR, Eshaghpour E, et al. The natural history of hypertrophic cardiomyopathy in infants of diabetic mothers. J Pediatr 1979; 95:1020.
- Royal Prince Alfred Hospital Newborn Guidelines – Polycythaemia: www.slhd.nsw.gov.au/rpa/neonatal/protocols.html.





good source of information