‘Hectic fever, at its inception, is difficult to recognise but easy to treat; left unattended it becomes easy to recognise and difficult to treat.’ Niccolo Machiavelli – historian, philosopher, humanist and Renaissance author (1469–1527)
Despite the many advances in medicine, maternal sepsis remains a leading cause of mortality. Prof Michael Humphrey quotes a six-fold reduction in maternal deaths from 1964–66 to 2008–12.1 In line with the Statement of Health by WHO,2 sepsis was identified as the third most frequent direct cause of maternal mortality in the world. In developed countries the incidence of maternal mortality from sepsis is far lower as it is largely preventable and more easily treated. This article aims to interpret and apply the new definition of sepsis and surviving sepsis guidelines to improve management of maternal sepsis in practice.
There have been numerous attempts at refining the definition of sepsis. The American College of Chest Physicians and the Society of Critical Care Medicine first developed a consensus definition of sepsis in 1991,3 which was further modified in 2001.4 Recognising the advances in the last decade, a taskforce consisting of experts from related specialities was convened to generate the Sepsis-3 definitions. The 2016 Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3)5 have replaced the earlier definitions. To reach a greater global consensus, WHO developed and proposed a new definition of maternal sepsis underpinning the medical concepts in Sepsis-3 definitions.6
Sepsis (as per Sepsis-3 definitions) is now defined as life-threatening organ dysfunction caused by a deregulated host response to infection. Organ dysfunction can be identified as an acute change in the total sepsis-related organ failure assessment score (SOFA) by 2 or more (Table 1). SOFA is a poor bedside tool as it requires laboratory investigations and may delay recognition of organ dysfunction. A new score called the qSOFA (quick SOFA) was introduced along with the new definition of sepsis that included altered mentation, systolic blood pressure of 100mmHg or less and respiratory rate of 22 or more. This provides a bedside tool to identify patients with infection who are likely to have a poor outcome. In addition, non-specific systemic inflammatory response syndrome (SIRS) criteria, such as pyrexia and leucocytosis, still aid in the diagnosis of infection.
Sepsis-3 defines septic shock as a subset of sepsis in which underlying circulatory and cellular abnormalities are profound enough to substantially increase mortality.7 Septic shock can be recognised with clinical construct of sepsis with persistent hypotension requiring vasopressors to maintain a mean arterial pressure (MAP) greater than 65mmHg and a serum lactate level of 2mmol or more per litre, despite adequate volume resuscitation.
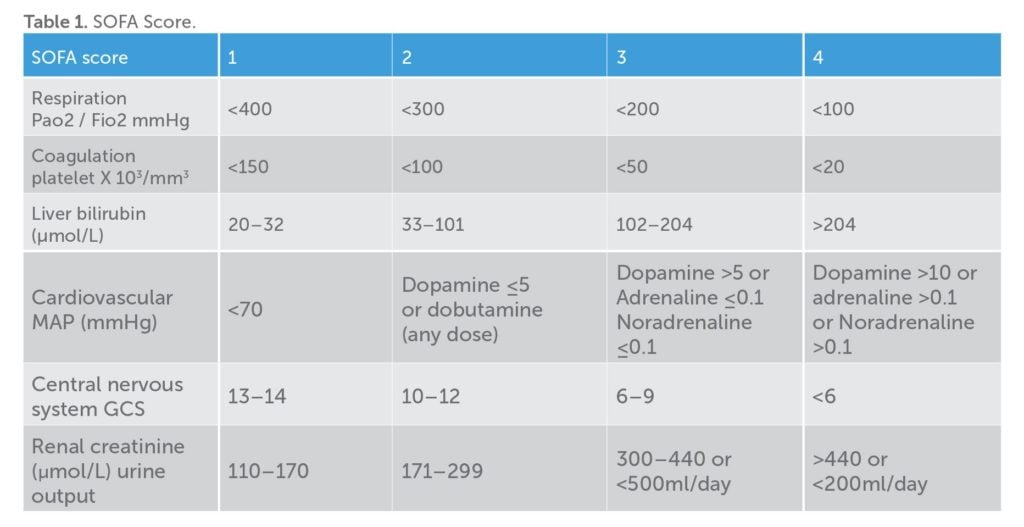
Based on Sepsis-3, WHO’s definition of maternal sepsis states that it ‘is a life-threatening condition defined as organ dysfunction resulting from infection during pregnancy, childbirth, post-abortion or postpartum period’.8 This new definition shifts the focus from inflammatory response to life-threatening organ dysfunction. Early recognition and treatment of infection can prevent organ failures. Healthcare professionals caring for pregnant women require a high degree of vigilance and skill to recognise early signs of sepsis.
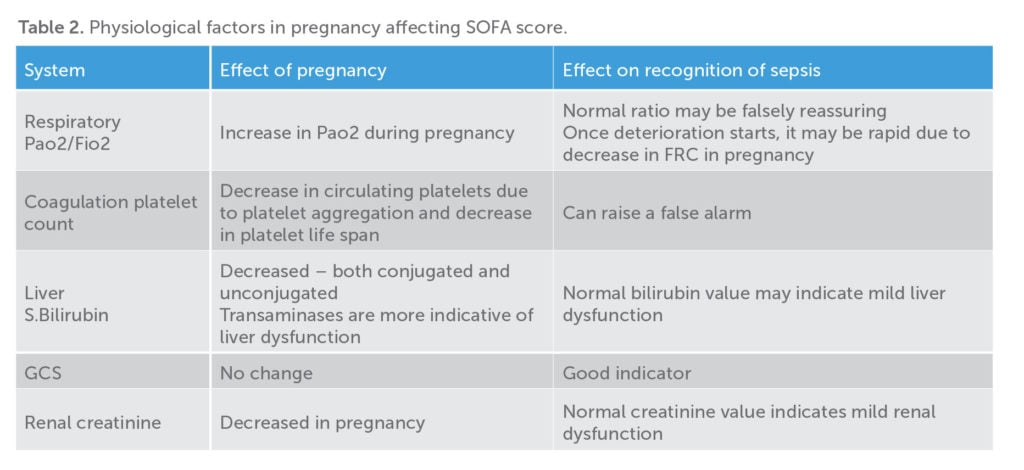
Onset of sepsis in pregnancy can be insidious and patients may appear deceptively normal before the development of organ dysfunction. Physiological changes in pregnancy can impact on both the SOFA and qSOFA scores, with diagnosis of sepsis often fraught with difficulties. A high index of suspicion has to be maintained, especially in the high-risk group. As per the Green-top Guideline 2012,9 the risk factors identified (by Confidential Enquiries into Maternal Deaths) are obesity, diabetes, anaemia, invasive procedures such as amniocentesis, cervical cerclage, prolonged spontaneous rupture of membranes, history of pelvic infections or group A streptococcus infection. Any patient who is lethargic, unwell looking with fever, tachypnoea, tachycardia, hypotension or drowsiness in pregnancy or postpartum should prompt the physician to initiate treatment urgently. The outcome of sepsis is improved by early detection, prompt recognition of source and targeted therapy.
Sepsis-3 recommends that once a patient with suspected infection meets two or more qSOFA criteria (bearing in mind the variations in pregnancy), the SOFA score should be calculated to diagnose sepsis. In practical terms, if more than two criteria of qSOFA are present, one should take cultures and consider antibiotics while assessing for organ dysfunction. With respect to calculating SOFA score, the physiological changes in pregnancy should be kept in mind (Table 2).
Aetiology of sepsis during pregnancy and postpartum can be obstetric or non-obstetric. The non-obstetric causes are similar to the general population and range from pyelonephritis and pneumonia to intraperitoneal pathologies. Obstetric-related causes include chorioamnionitis, endomyometritis, pelvic abscess, septic abortion and necrotising fasciitis from wounds, to name a few. Specific symptoms act as a guide in selecting the sites for microbiological sampling.
Management of sepsis and septic shock
Initial resuscitation
Sepsis and septic shock are medical emergencies and resuscitation should commence immediately. There is no evidence for early goal-directed therapy in sepsis (ARISE10, ProCESS11 AND ProMISe12). The Surviving Sepsis Campaign recommends using initial fluid resuscitation of 30ml per kilo of crystalloid within the first three hours while more specific information is obtained and hemodynamic assessment is made.13 An initial target is to attain a MAP of greater than 65mmHg and normalise serum lactate as it is a marker of tissue perfusion. Vasopressor therapy (noradrenaline as the first choice) should be considered if unable to achieve a MAP of greater than 65mmHg with fluid resuscitation.
Antimicrobial therapy
Blood and microbiological cultures from potential sources must be obtained prior to initiating antimicrobials. The antimicrobials should be administrated within the first hour of suspecting sepsis.14 There should be no more than a 45-minute delay in obtaining cultures to facilitate early antimicrobial therapy.15 Broad-spectrum empiric intravenous antimicrobials should be chosen in accordance with local antimicrobial guidelines. Antibiotic therapy should be de-escalated once the pathogen is identified and sensitivities established.
Indications to move to high dependency unit
Persistent shock despite fluid therapy, respiratory failure, acute renal failure, altered consciousness, multi-organ dysfunction and metabolic derangements are indications to transfer patient to a high dependency unit environment.16 Central lines and arterial line should be placed for all patients requiring vasopressors as soon as practical.
Source control
Appropriate intervention to control the source of sepsis should be implemented as soon as medically and logistically practical. Removal of retained products of conception, debridement of wound infections and drainage of abscesses should be done without delay. Early general surgical help should be sought for any other suspected non-obstetric surgical complications.
Fetal monitoring
In critically ill pregnant women with sepsis, stabilising the mother is priority. Once in an ICU environment the obstetric team should liaise with the ICU team to plan fetal monitoring and delivery time. Continuous CTG monitoring is recommended. Attempting delivery in the setting of maternal instability increases both maternal and fetal mortality.17
Supportive care for patients in ICU
Venous thromboembolism prophylaxis with low-molecular-weight heparin, stress ulcer prophylaxis, adequate nutrition, correction of electrolytes, prevention of pressure sores and family counselling should be embedded in the care of a critically ill obstetric woman.
Adjuvant therapies for sepsis and septic shock
If adequate resuscitation and vasopressor therapy fails to restore haemodynamic stability, the Surviving Sepsis Campaign Guidelines suggest 200mg of intravenous hydrocortisone per day.18 Intravenous immunoglobulin (IVIG) is not indicated in septic shock. Use of IVIG in toxic shock due to staphylococcal and streptococcal infection should be discussed with infectious disease specialists. Glycaemic control with a protocol-based approach to maintain a blood glucose level below 10mmol per litre is recommended.
Infection prevention
Poor hand hygiene was a recognised cause of spread of puerperal fever in the early 19th century. In 1847, Semmelweis from Austria asked his students to wash their hands with chlorine before examination, which led to a striking decrease in mortality.19 Today, multidrug-resistant organisms causing hospital-acquired infections have become a major concern. Hand hygiene is the most important infection-prevention technique. A strict adherence to personal protective equipment guidelines is mandatory. Local hospital guidelines should be followed for isolation and contact precaution.
Healthcare workers exposed to group A streptococcal infection should be referred to infectious disease specialists for consideration of antibiotic prophylaxis and the department of health should be notified. A neonatologist should be consulted regarding prophylaxis for the baby.
Summary
Severe sepsis and septic shock are a life-threatening immune response to infection that causes injury to one’s own tissues and organs. Early recognition, multidisciplinary approach and prompt treatment decreases mortality and morbidity. Rigorous adherence to sepsis and infection prevention guidelines will help decrease spread of infections.
References
- AIHW: Humphrey MD, Bonello MR, Chughtai A, et al. Maternal deaths in Australia 2008–2012. Maternal deaths series no.5. Cat. no. PER 70. Canberra: AIHW. 2015.
- World Health Organization: Statement on Maternal Sepsis.WHO. 2017. Available from: http://apps.who.int/iris/ bitstream/10665/254608/1/WHO-RHR-17.02-eng.pdf?ua=1.
- Members of the American College of chest Physicians/Society of Critical Care Medicine Consensus. Conference Committee. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864-74.
- Levy MM, Fink MP, Marshall JC, et al. SCCM/ESICM/ACCP/ATS/ SIS International Sepsis Definitions Conference. Crit Care Med. 2001;31:1250-6.
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definition for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-10.
- World Health Organization: Statement on Maternal Sepsis. WHO. 2017. Available from: http://apps.who.int/iris/ bitstream/10665/254608/1/WHO-RHR-17.02-eng.pdf?ua=1.
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definition for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-10.
- World Health Organization: Statement on Maternal Sepsis. WHO. 2017. Available from: http://apps.who.int/iris/ bitstream/10665/254608/1/WHO-RHR-17.02-eng.pdf?ua=1.
- Royal College of Obstetricians and Gynaecologists. Bacterial Sepsis in Pregnancy. Green-top Guideline No. 64a. RCOG. 2012. Available from: www.rcog.org.uk/globalassets/documents/ guidelines/gtg_64a.pdf.
- ARISE Investigators, Anzics Clinical Trials Group, Peake SL, Delaney A, Bailey M, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496-506.
- Process Investigators, Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683-93.
- Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372(14):1301–11.
- Rhodes A, Evans LE, Waleed A, et al; Surviving Sepsis Campaign: International Guidelines for management of Sepsis and Septic shock: 2016. Critical Care Med. 2017;45(3):486-552.
- Kumar A, Roberts D, Woods KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Critical Care Med. 2006;34(6):1589-1596.
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definition for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-10.
- Royal College of Obstetricians and Gynaecologists. Bacterial Sepsis in Pregnancy. Green-top Guideline No. 64a. RCOG. 2012. Available from: www.rcog.org.uk/globalassets/documents/ guidelines/gtg_64a.pdf.
- Royal College of Obstetricians and Gynaecologists. Bacterial Sepsis in Pregnancy. Green-top Guideline No. 64a. RCOG. 2012. Available from: www.rcog.org.uk/globalassets/documents/ guidelines/gtg_64a.pdf.
- Rhodes A, Evans LE, Waleed A, et al; Surviving Sepsis Campaign: International Guidelines for management of Sepsis and Septic shock: 2016. Critical Care Med. 2017;45(3):486-552.
- Carmen D, Claudia S. Global burden of maternal sepsis in the year 2000. Evidence and Information for Policy. WHO, Geneva. 2003. Available from: www.who.int/healthinfo/statistics/bod_ maternalsepsis.pdf.





Leave a Reply