The patient was a 36-year-old mother of two, referred in 2016 with a provisional diagnosis of genital graft versus host disease (GVHD). In 2004, four months after the birth of her youngest child, she developed myeloid leukaemia treated successfully with total body irradiation, chemotherapy and subsequent bone marrow transplant. The following year she developed vaginal soreness and fissuring precluding intercourse, which was diagnosed as GVHD. Treatment with vaginal oestrogen and the oral contraceptive pill was of little or no benefit. She appeared fit, with a good recovery and her only complaints apart from vaginal soreness are amenorrhoea, dry eyes, partial lung capacity and partial return of immunity.
Somewhat surprisingly, her vulva appeared normal, with evidence of fourchette fissuring only, and no apparent lichenoid dermatosis, the usual feature of GVHD.1 She did, however, have a clinically apparent vaginitis with slight stenosis of the upper one-third of the vagina. Bimanual findings were normal.
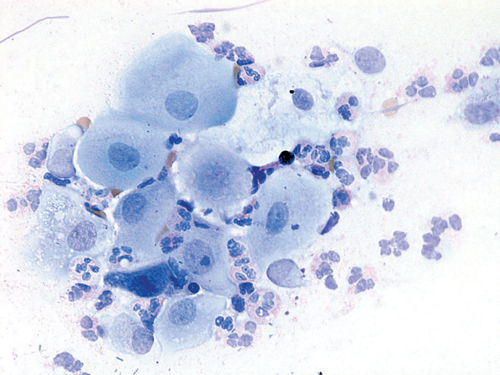
Figure 1. Vaginal smear at initial presentation showing severe vaginitis and parabasal cells (Wright oestrogen is its influence on the regulation
stain x 400).
A stained smear from the vagina consisted mainly of inflammatory cells and parabasal cells, indicative of severe atrophic and/or erosive vaginitis (Figure 1). Her serum oestradiol was unrecordable and her follicle-stimulating hormone (FSH) was 184 IU/L.
She was commenced on oral oestradiol 2mg and medroxyprogesterone acetate 5mg daily and no topical treatment. At review 17 days later, there was relief of genital symptoms. A stained smear from the vagina this time revealed resolution of the vaginitis and consisted almost totally of healthy superficial and intermediate squamous cells (Figure 2).
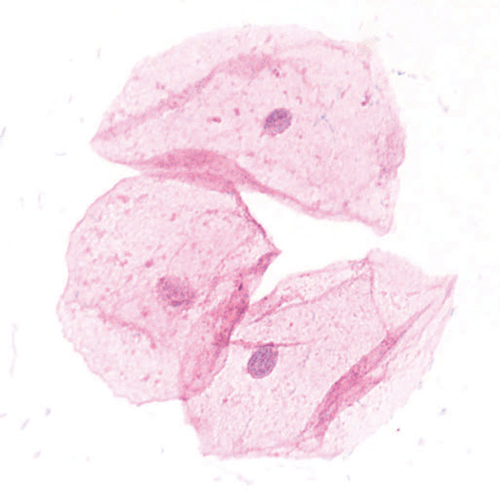
Figure 2. Vaginal smear 17 days after treatment showing resolution of vaginitis and epithelial maturity (Wright stain x 400).
We present a case with a provisional diagnosis of GVHD with an excellent response to systemic oestrogen replacement. While the first smear findings could have been due to atrophy and/or vaginitis (erosive/autoimmune), her vaginitis and stenosis were typical signs of GVHD and her marked improvement with oestrogen alone suggested lack of oestrogen was her main problem. Her subsequent progress, however, confirmed GVHD was also present.
Oestrogen improves symptoms in vaginal GVHD2 and not all patients with vulvovaginal GVDH require immunosuppresive therapy. Treatment with oestrogen replacement alone at an optimal dosage avoids the increased risk of local infectious complications, especially fungal infections, that may follow topical steroids. One mechanism for the improvement with oestrogen is its influence on the regulation of the immune system of the lower female genital tract.3 Despite her improvement with oestrogen alone, the patient remains under close surveillance in case of relapse of her GVHD, as prompt diagnosis and appropriate therapy may prevent the development of severe forms of GVHD and the need for surgery.
The treatment of vaginal atrophy is somewhat controversial.4 5 It is almost always due to oestrogen lack and replacement of oestrogen is the usual treatment. One author (GD) has a strong preference for systemic oestrogen replacement6 because of complications of vaginal oestrogen, including dosage difficulties and their consequences and contact dermatitis from prolonged usage. Furthermore, the woman relying on topical replacement misses out on the benefits of systemic menopausal hormone therapy (MHT), such as the prevention of osteoporosis as well as relief of any other menopausal symptoms. This case lends further weight to the contention that the best treatment for atrophic vaginitis is systemic oestradiol, which can be monitored if necessary with a serum oestradiol and FSH. A serum oestradiol over 150pmol/L guarantees vaginal oestrogenisation.7
Follow up
The patient returned 20 weeks after the initial consultation, still taking MHT, complaining of a sensation of dryness and ‘splitting’ affecting the vulva. Examination revealed minimal inflammatory changes only, but in view of the complexity of the case, a biopsy was taken from the interlabial sulcus from where most of her discomfort arose. The histology (Figures 3 and 4) was indicative of GVHD with the characteristic ‘lichenoid’ features. She was rendered free of symptoms and signs with topical 1% hydrocortisone.
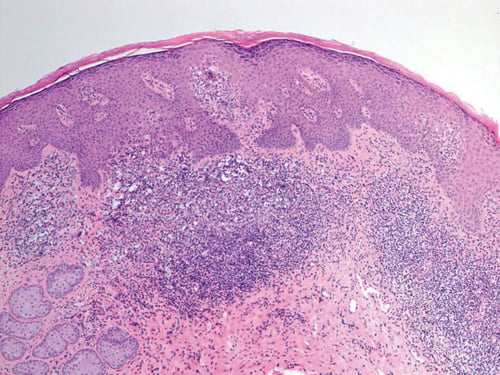
Figure 3. Subsequent biopsy interlabial sulcus.
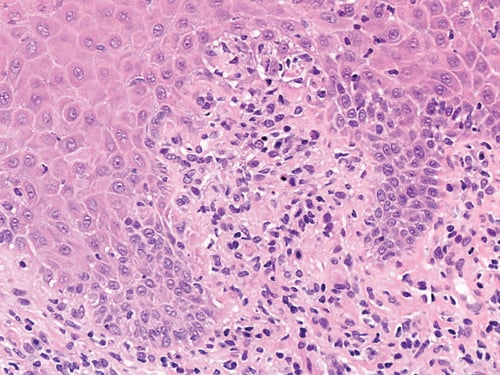
Figure 4. Subsequent biopsy interlabial sulcus.
The management of this rather complex case was expedited by the use of office microscopy by one of the authors (GD) and adds support for the ongoing teaching of this technique, particularly to those with a special interest in vulvovaginal disorders.8
References
- Wolff D, Gerbitz A, Ayuk F, et al. Consensus conference on clinical practice in chronic graft-versus-host disease (GVHD): first-line and topical treatment of chronic GVHD. Biol Blood Marrow Transplant. 2010;16(12):1611-28.
- Wolff D, Gerbitz A, Ayuk F, et al. Consensus conference on clinical practice in chronic graft-versus-host disease (GVHD): first-line and topical treatment of chronic GVHD. Biol Blood Marrow Transplant. 2010;16(12):1611-28.
- Beagley KW, Gockel CM. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol Med Microbiol. 2003;
38(1):13-22. - Santoro N, Worsley R, Miller KK, Parish SJ, Davis SR. Role of Estrogens and Estrogen-Like Compounds in Female Sexual Function and Dysfunction. J Sex Med. 2016;13(3):305-16.
- Mac Bride MB, Rhodes DJ, Shuster LT. Vulvovaginal Atrophy. Concise review for clinicians. Mayo Clin Proc. 2010;85(1): 87-94.
- Dennerstein G. Re: Hormones down under: Hormone therapy use after the Women’s Health Initiative. Letter to the Editor. ANZJOG. 2007;47:1:80.
- Dennerstein G. Re: Hormones down under: Hormone therapy use after the Women’s Health Initiative. Letter to the Editor. ANZJOG. 2007;47:1:80.
- The Vulva & Vaginal Manual. Dennerstein G, James Scurry, John Brenan, David Allen & Maria-Grazia Marin. Gynederm Publishing, Melbourne 2005.






Leave a Reply