Genetic counselling is the communication process that helps patients understand and adapt to the implications of health conditions that have a genetic contribution. Genetic counselling should take place, at least to some extent, in the context of all pregnancies. The ideal time is before conception, but when this is not possible, early pregnancy is preferable. Complex cases, such as when there is a known family history of a hereditary disorder, are best referred to a clinical genetics service; however, most pre-pregnancy genetic counselling is performed by general practitioners, midwives and obstetricians. The key components of genetic counselling are summarised in Table 1.
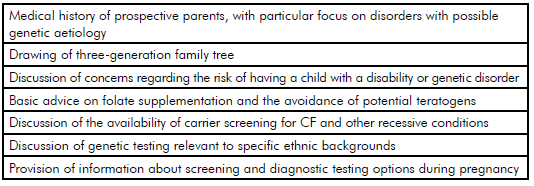
Table 1. Essential components of pre-pregnancy genetic counselling.
Pre-pregnancy genetic counselling should include open-ended enquiry about any concerns that the prospective parents may have about having a child with a genetic condition or congenital abnormality. In some situations, such concerns may be unfounded and reassurance may be all that is required. Alternatively, further enquiry and investigation may be necessary. It is important to recognise that attitudes of prospective parents towards disability and prenatal testing vary greatly; it is helpful to clarify the views and beliefs of the prospective parents early in the counselling process.
It is also important to recognise that some congenital abnormalities are not genetic in origin. In addition, many disorders that are genetic are not inherited from a parent; but rather occur as the result of new gene mutations arising in the child. This highlights that prior to pregnancy it is not possible to predict, or prevent, all genetic disorders or congenital abnormalities. Table 2 provides a summary of the types of genetic disorders that are potentially identifiable before pregnancy. The availability of pre-implantation genetic diagnosis (PGD) now provides a greater incentive to identify at-risk couples prior to pregnancy.
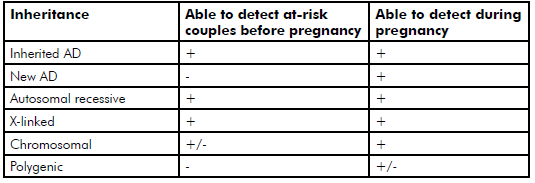
Table 2. Types of genetic disorder that are potentially identifiable before pregnancy.
Despite the increasing profile of new, powerful genetic-testing technologies, the personal and family histories of the prospective parents remain an important screening tool. It is helpful to draw a
three-generation family tree, and ask focused questions about any history of genetic disorders, stillbirths, miscarriages, consanguinity and the birth of children with physical or intellectual disability. A history of affected males on the maternal side of the family is a warning sign that an X-linked disorder may be present.
The pedigree may indicate the presence, or raise suspicion, of a genetic disorder in the family, particularly if there is evidence of autosomal dominant (AD) or X-linked transmission. Common examples of AD disorders include neurofibromatosis, Marfan syndrome and familial cancer syndromes (for example, familial breast and ovarian cancer caused by mutations in the genes BRCA1 and BRCA2). Frequently encountered X-linked disorders are Fragile X syndrome, haemophilia A and Duchenne muscular dystrophy. Autosomal recessive disorders, such as cystic fibrosis (CF), are seldom detectable by family history, but a family history of the more common autosomal recessive disorders should prompt consideration of carrier testing in the couple.
Some prospective parents may raise concerns about the risk of a disorder that has complex, or polygenic, inheritance, particularly if one of the parents is affected by the condition. Examples include autoimmune disease, diabetes and major psychiatric disorders. Genetic testing is usually not helpful in this setting; however, empiric risk figures are available and can be used in counselling. Even when one parent is affected by the polygenic condition, the risk to their offspring is typically low (less than five per cent).
Frequently, prospective parents are concerned about the effect of their age on the risk of having a child with a genetic disorder. Most women have a good understanding that the risk of having a baby with Down syndrome increases with maternal age, and that there is a parallel increase in the risks of miscarriage and of other chromosome aneuploidies. There is also increasing recognition that the risk of single gene disorders, caused by de novo gene mutations, increases with paternal age. In recent years, it has become evident that a substantial proportion of genetic conditions occur as a result of de novo mutations, examples of which include achondroplasia and craniosynostosis syndromes.
Genetic carrier screening
Autosomal recessive disorders occur when both parents are carriers of a faulty gene that, when present in a ‘double dose’ in a child, results in the disorder. There are literally thousands of autosomal recessive conditions and, in the vast majority of cases, an affected child is born into a family with no family history of the condition. It is now recognised that all people are carriers of multiple recessive genes; these cause no harm to the individual, but pose a risk if their partner carries a fault in the same recessive gene. Of course, statistically, this is unlikely to occur in an individual couple, but collectively autosomal recessive disorders result in very significant morbidity and mortality. Therefore, there is considerable interest in whether these disorders can be prevented by identifying at-risk couples prior to pregnancy.
The best-studied example of population-based carrier screening in Australia is CF. CF is the most common severe autosomal recessive disorder in the Australian population and one-in-25 Caucasians are carriers. Importantly, 94 per cent of babies with CF are born into families where there is no known family history and so a screening approach where only those with a family history of CF are offered testing is not sufficient.1 Population screening (offering carrier testing to prospective parents regardless of family history) for CF is made more straightforward by the fact that a small number of recurrent gene mutations account for most children born with CF, and has been available in Australia for more than ten years, with strong endorsement from the CF support groups and the Human Genetics Society of Australasia.2 Usually CF population screening is performed as a two-step process: one partner is screened first, and the other partner is tested only if a mutation is detected in the first partner. This process has been shown to be effective in identifying carrier couples, and most carrier couples detected choose either preimplantation genetic diagnosis or prenatal diagnosis by chorionic villus sampling.3 Similar outcomes have been demonstrated for thalassaemia screening4 and for Tay-Sachs disease screening in the Ashkenazi Jewish community.5
Expanded carrier screening
Although carrier screening for common recessive disorders (such as CF and thalassaemia) is of proven benefit, the overall impact of screening is limited by the fact that these disorders represent only a small fraction of the thousands of rare autosomal recessive disorders. It is now known that we are all carriers of approximately five to ten autosomal recessive disorders; if this carrier status could be detected, then the overall health burden of autosomal recessive disease might be reduced substantially. The advent of next-generation gene sequencing technology offers the potential to extend the CF screening model to include hundreds or even thousands of diseases.6
During the last five years, proof-of-principle studies of expanded carrier screening have been published7 8, and commercial screening tests are currently being offered in Australia. Expanded carrier screening tests typically offer screening for more than 100 different recessive disorders, with selection of disorders for testing based on their prevalence, seriousness and the ability of carrier status to be determined with accuracy.9 10 Testing for Fragile X syndrome, the most common inherited form of intellectual disability, is added as a separate test because it requires a different testing technology. Although there are clear potential benefits of these tests, their clinical utility is currently limited by lack of knowledge regarding the pathogenicity of many sequence variants. As a result, for some of the conditions tested, the sensitivity of these tests (in other words, the proportion of carrier couples detected by the test) is less than ten per cent. Other criticisms of these tests include the lack of supportive guidelines from professional organisations, marketing of tests directly to patients, the cost of follow-up testing and counselling and the absence of public funding.11 12 In spite of these valid concerns, as the technology matures over the next decade, it is likely that these tests will become widely used, and may eventually become standard care.
Concluding comments
All couples hope for a healthy baby, and the advent of new genetic-testing technologies provides the opportunity to reduce the risk of having a child with a serious disability. The evolution of these new tests means that pre-pregnancy counselling is more important, but also more complex, than ever before.
References
- McClaren BJ, Metcalfe SA, Amor DJ et al. A case for cystic fibrosis carrier testing in the general population. Medical Journal of Australia. 2011; 194(4):208-9.
- Delatycki M, Burke J, Christie L et al. Human Genetics Society of Australasia Cystic Fibrosis Population Screening Position Paper. HGSA; 2009.
- Archibald AD, Massie J, Smith MJ et al. Population-based genetic carrier screening for cystic fibrosis in Victoria. Medical Journal of Australia. 2014; 200(4):205-6.
- Tan YL, Kidson-Gerber G. Antenatal haemoglobinopathy screening in Australia. Medical Journal of Australia. 2016;204(6):226-30.
- Lew RM, Proos AL, Burnett L et al. Tay Sachs disease in Australia: reduced disease incidence despite stable carrier frequency in Australian Jews. Medical Journal of Australia. 2012; 197(11):652-4.
- Lazarin GA, Goldberg JD. Current controversies in traditional and expanded carrier screening. Current Opinion in Obstetrics & Gynecology. 2016; 28(2):136-41.
- Bell CJ, Dinwiddie DL, Miller NA, Hateley SL, Ganusova EE, Mudge J et al. Carrier testing for severe childhood recessive diseases by next-generation sequencing. Science Translational Medicine. 2011; 3(65):65ra4.
- azarin GA, Haque IS, Nazareth S et al. An empirical estimate of carrier frequencies for 400+ causal Mendelian variants: results from an ethnically diverse clinical sample of 23,453 individuals. Genetics in Medicine: Official Journal of the American College of Medical Genetics. 2013;15(3):178-86.
- Lazarin GA, Haque IS, Nazareth S et al. An empirical estimate of carrier frequencies for 400+ causal Mendelian variants: results from an ethnically diverse clinical sample of 23,453 individuals. Genetics in Medicine: Official Journal of the American College of Medical Genetics. 2013;15(3):178-86.
- Edwards JG, Feldman G, Goldberg J et al. xpanded carrier screening in reproductive medicine-points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstetrics and Gynecology. 2015; 125(3):653-62.
- Edwards JG, Feldman G, Goldberg J et al. xpanded carrier screening in reproductive medicine-points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstetrics and Gynecology. 2015; 125(3):653-62.
- Stoll K, Resta R. Considering the cost of expanded carrier screening panels. Genetics in Medicine: Official Journal of the American College of Medical Genetics. 2013; 15(4):318-9.







Leave a Reply