Data from the influenza pandemic of 2009 confirmed known observations from previous seasonal epidemics and pandemics: pregnant women are at increased risk for severe influenza virus infection. Higher rates of hospitalisation, fetal loss and maternal death are well-recognised complications of influenza virus infection in pregnancy, particularly during the late gestational period. The safe and immunogenic influenza vaccine is the only proven method to prevent influenza infection and should be prioritised in pregnant women.
Influenza viruses are single stranded, negative sense RNA viruses belonging to the family Orthomyxoviridae. Within this family, influenza virus types A and B primarily cause human infection. Influenza virus infection occurs when the surface glycoprotein haemagglutinin (H) binds the sialic acid- containing receptors on target host cells to initiate infection. Following viral replication, neuraminidase (N) cleaves sialic acids from cellular receptors and extracellular inhibitors to facilitate progeny release and spread to neighbouring cells (see Figure 1).
Influenza A viruses are also subtyped based on the haemagglutinin and neuraminidase glycoproteins, for example, influenza A/ H1N1, H3N2 and avian influenza A/H5N1 and H7N9. A remarkable feature of influenza viruses lies in their ability to undergo antigenic variation. Antigenic drifts result from point mutations in the haemagglutinin and neuraminidase genes, which allow the virus to evade the human immune response, resulting in seasonal epidemics. This potentially necessitates annual changes inthe influenza vaccine. Antigenic shifts occur when a new influenza subtype emerges, or from the reassortment of haemagglutinin and neuraminidase genes between human and non-human (avian or swine) subtypes. Owing to the lack of pre-existing cross-protective antibodies in susceptible populations, global epidemics or pandemics result.
The burden of influenza infection is typically underestimated as symptoms of influenza are not specific to influenza virus per se and infection may not be laboratory confirmed. In Australia, modelling suggests that 310 000 general practice consultations and 18 400 hospitalisations result from influenza infection annually, at an estimated cost of $115 million.1 In New South Wales, influenza and pneumonia were responsible for 9.1 per cent of total deaths in 2013 (available at: www.health.nsw.gov.au/Infectious/Influenza/ Documents/2013/december-report.pdf).
Severe influenza infection in pregnant women was first noted during the influenza pandemic of 1918, with reported mortality rates of 27–45 per cent. This has also been consistently observed in subsequent pandemics, in which infection in pregnant women was associated with an increased risk of both severe complications and death.2 3 4 5 During the influenza A pandemic of 2009, 9.1 per cent of patients admitted to intensive care units (ICUs) in Australasia were pregnant (approximately ten times the incidence of pregnancy in the community) and mortality rates were approximately 8.6 per cent.6 In Victoria, 51 per cent of pregnant women with complicated influenza had an additional comorbidity, including asthma, smoking and/or diabetes, but the remainder had no additional risk factor for severe influenza infection.7
Poor outcomes from influenza infection in pregnancy are not attributable to anatomical and physiological changes in the respiratory tract alone (such as reduced functional residual capacity, increased oxygen consumption or progesterone induced hyperventilation). Pregnancy is associated with a generalised state of immune tolerance or immunosuppression, with increased severity and susceptibility to infections including, but not limited to, listeriosis, malaria and hepatitis E virus. Pregnancy is also associated with a bias towards Th2-associated humoral immunity as a predominant Th1 cell-mediated immune response is harmful to the fetus, characterised by low peripheral natural killer cell activity.8 Of note, there is increased expression of pro-inflammatory cytokines such as IL-6, IL-8 and TNF-α following maternal influenza virus infection.9
The direct effects of maternal influenza infection on the fetus are not well studied or understood at present. Maternal viraemia and transplacental transmission following influenza infection are uncommon, yet adverse fetal outcomes in the absence of direct fetal infection have been observed.10 Increased rates of spontaneous miscarriage, low birthweight, preterm delivery and fetal death have been observed and reported, mainly during influenza pandemics.11 12 13 14 A recent review of non-chromosomal congenital abnormalities noted the increased risk of hydrocephaly, cleft lip, neural tube defects and congenital heart defects following maternal influenza infection or influenza-like illness during the first trimester.15 Another potential added risk of influenza infection is maternal hyperthermia, known to have an association with neural tube defects.16 17 However,this risk is mitigated by the relatively short duration of fever and response to antipyretics in influenza infection.
Influenza virus is easily spread from person to person via coughing and sneezing, resulting in small-particle aerosols that can remain suspended in the air for many hours, or by direct contact with an infected individual or environments contaminated with respiratory secretions. Clinical manifestations of influenza infection in pregnant women are similar to those of the general population. After an incubation period of one to three days, typical symptoms include fever, sore throat, rhinorrhoea, cough, shortness of breath and myalgia. Infection can range from mild and self-limiting to severe and life-threatening when complicated by primary viral pneumonitis or secondary bacterial pneumonia and other less common complications such as kidney injury and encephalopathy.
Laboratory confirmation of influenza guides treatment, obviates the need for further unnecessary testing and informs infection control procedures and vaccination strategies.18 When selecting the most appropriate test, clinicians should consider the availability, performance and turnaround times of the different diagnostic methods, such as antigen detection, viral culture and nucleic acid tests (NATs). Influenza diagnostic tests can be performed on upper and lower respiratory tract samples, including nose and throat swabs, nasopharyngeal swabs, bronchoalveolar lavage fluid and pleural fluid. Sputum is not a preferred specimen owing to its viscosity.
The sensitivity of influenza diagnostic tests may be increased when lower respiratory tract samples are tested. The detection of viruses from respiratory samples is also affected by the time between symptom onset and specimen collection. Respiratory viruses are more likely to be detected when specimens are collected soon after symptom onset, as viral loads are generally higher early in the illness. The quality of sample collection is especially important in respiratory tract infection and training in sampling is recommended. Self- collected mid-turbinate nasal swabs may be considered to reduce turnaround times, although data on the sensitivity and specificity of testing using this approach are awaited.19
Antigen detection-based rapid influenza diagnostic tests (RIDTs) are simple to perform and interpret, but have limited sensitivity and specificity in detecting influenza virus, particularly when influenza activity is low or for novel influenza subtypes.20 Although viral culture remains the gold standard for diagnosis, NATs are predominantly used given their increased sensitivity, specificity, breadth and reduced turnaround time to pathogen detection. Multiplex polymerase chain reaction assays allow the simultaneous detection of different respiratory viruses and some assays also contain bacterial targets.
Antiviral therapy for influenza virus infection should be initiated within 48 hours of symptom onset. However, hospitalised patients (including pregnant women) may benefit from antiviral therapy even when commenced after 48 hours. A four-fold increase in the risk of ICU admission or death was noted when the institution of oseltamivir was delayed beyond 48 hours after initial presentation.21 Empirical antiviral therapy should not be delayed if influenza is suspected before laboratory confirmation of infection. Despite the benefits of oseltamivir (a category B1 medication in pregnancy), only 50–81 per cent of hospitalised pregnant women received this medication during the influenza pandemic of 2009.7,22 23 There is reluctance among pregnant women to use oseltamivir despite available post-marketing data suggesting its safety during pregnancy and the benefits outweighing the potential risks to the fetus.24 Women who are up to two weeks postpartum with suspected or confirmed influenza should also be treated with antiviral therapy.25
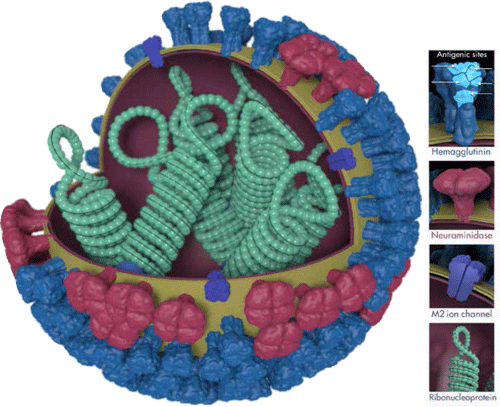
Figure 1. Influenza virus, illustrating the surface glycoproteins haemagglutinin and neuraminidase. Illustration courtesy of Dan Higgins, CDC Public Health Image Library.
Influenza vaccination is the only proven method to prevent influenza virus infection and is recommended for all pregnant women. Furthermore, it protects infants aged less than six months, in whom influenza vaccination in contraindicated.26 27 Influenza vaccines may be administered an any time during pregnancy, before or during the influenza season. No significant long-term side effects (teratogenic, carcinogenic or neurologic) have been shown in pregnant women receiving the influenza vaccine during the first trimester3 and would not be expected from a killed vaccine. A cohort of 6989 infants exposed to an adjuvanted influenza A(H1N1)pdm09 vaccine during pregnancy was not associated with increased risk of major birth defects, preterm birth or fetal growth restriction.28 However, the uptake of the vaccine in pregnant women, even in the later stages of pregnancy is universally poor, despite evidence of significantly increased morbidity and mortality from influenza infection.7 A recent Australian study also revealed that one-third of general practitioners did not believe influenza infection to be a significant risk to mother or baby and more than half had significant concerns about the safety of influenza vaccination during pregnancy.29
In conclusion, influenza virus infection during pregnancy causes significant morbidity and mortality. While further understanding of the pathogenesis of infection during pregnancy may help identify potential targets for therapies, vaccination is safe and effective and should be prioritised in this at-risk group to prevent infection.
References
- Newall AT, Scuffham PA. Influenza-related disease: the cost to the Australian healthcare system. Vaccine 2008; 26: 6818-6823.
- Harris JW. Influenza occurring in pregnant women: a statistical study of thirteen hundred and fifty cases. JAMA 1919; 72: 978-980.
- Mak TK, Mangtani P, Leese J, Watson JM, Pfeifer D. Influenza vaccination in pregnancy: current evidence and selected national policies. Lancet Infect Dis 2008; 8: 44-52.
- Titus P, Jamison JM. Pregnancy complicated by epidemic influenza. JAMA 1919; 72:1665-1668.
- Eickhoff TC, Sherman IL, Serfling RE. Observations on excess mortality associated with epidemic influenza. JAMA 1961; 176: 776-782.
- The ANZIC Influenza Investigators. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med 2009; 361: 1925-1934.
- Hewagama S, Walker SP, Stuart RL, et al. 2009 H1N1 influenza A and pregnancy outcomes in Victoria, Australia. Clin Infect Dis 2010; 50: 686–690.
- Szekeres-Batrho J. Immunological relationship between the mother and the fetus. Int Rev Immunol 2002; 21: 471-495.
- Periolo N, Avaro M, Czech A, et al. Pregnant women infected with pandemic influenza A(H1N1)pdm09 virus showed differential immune response correlated with disease severity. J Clin Virol 2015; 64: 52-58.
- Irving WL, James DK, Stephenson T, et al. Influenza virus infection in the second and third trimesters of pregnancy: a clinical and seroepidemiological study. BJOG 2000; 107: 1282-1289.
- Håberg SE, Trogstad L, Gunnes N, et al. Risk of fetal death after pandemic influenza virus infection or vaccination. N Engl J Med 2013; 368: 333-340.
- Mosby LG, Rasmussen SA, Jamieson DJ. 2009 pandemic influenza A (H1N1) in pregnancy: a systematic review of the literature. Am J Obstet Gynecol 2011; 205: 10-18.
- Centers for Disease Control and Prevention. Maternal and infant outcomes among severely ill pregnant and postpartum women with 2009 pandemic influenza A (H1N1)–United States, April 2009-August 2010. Morb Mortal Wkly Rep 2011; 60: 1193-1196.
- McNeil SA, Dodds LA, Fell DB, et al. Effect of respiratory hospitalization during pregnancy on infant outcomes. Am J Obstet Gynecol 2011; 204: S54-S57.
- Luteijn JM, Brown MJ, Dolk H. Influenza and congenital anomalies: a systematic review and meta-analysis. Hum Reprod 2014; 29: 809-823.
- Moretti ME, Bar-Oz B, Fried S, Koren G. Maternal hyperthermia and the risk for neural tube defects in offspring: systematic review and meta-analysis. Epidemiol 2005; 16: 216-219.
- Edwards MJ. Review: hyperthermia and fever during pregnancy. Birth Defects Res A Clin Mol Teratol 2006; 76: 507-516.
- Somerville LK, Ratnamohan VM, Dwyer DE, Kok J. Molecular diagnosis of respiratory viruses. Pathology 2015; 47: 243-249.
- Thompson MG, Ferber JR, Odouli R, et al. Results of a pilot study using self-collected mid-turbinate nasal swabs for detection of influenza virus infection among pregnant women. Influenza Other Respir Viruses 2015. doi: 10.1111/irv.12309. [Epub ahead of print].
- Kok J, Blyth CC, Foo H, et al. Comparison of a rapid antigen test with nucleic acid testing during cocirculation of pandemic influenza A/H1N1 2009 and seasonal influenza A/ H3N2. J Clin Microbiol 2010; 48: 290- 291.
- Louie J, Acosta M, Jamieson D, Honein M. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med 2010; 362: 27-35.
- Louie J, Acosta M, Jamieson D, Honein M. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med 2010; 362: 27-35.
- Jamieson D, Honien M, Rasmussen S, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 2009; 374: 451-458.
- Wollenhaupt M, Chandrasekaran A, Tomianovic D. The safety of oseltamivir in pregnancy: an updated review of post- marketing data. Pharmacoepidemiol Drug Saf 2014; 23: 1035-1042.
- Centers for Disease Control and Prevention.Recommendations for obstetric health care providers related to use of antiviral medications in the treatment and prevention of influenza (Available at: www.cdc/gov/flu/professionals/antivirals/avrec_ob.htm, accessed 16 April 2015).
- Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008; 259: 1555-1564.
- Steinhoff MC, Omer SB. A review of fetal and infant protection associated with antenatal influenza immunization. Am J Obstet Gynecol 2012; 207 S21-S27.
- Pasternak B, Svanstrom H, Molgaard- Nielsen D, et al. Risk of adverse fetal outcomes following administration of a pandemic influenza A(H1N1) vaccine during pregnancy. JAMA 2012; 308: 165-174.
- Maher L, Dawson A, Wiley K, et al. Influenza vaccination during pregnancy: a qualitative study of the knowledge, attitudes, beliefs, and practices of general practitioners in Central and South-Western Sydney. BMC Family Practice 2014; 15: 102.








Leave a Reply