Fetal surveillance and timing of delivery in the prevention of stillbirth.
Stillbirth is a global human tragedy, with approximately three million stillbirths occurring each year.1 While the majority of these occur in the developing world2, even in Australia, one in 130 families will experience the devastating outcome of stillbirth (defined as death before or during birth after 20 weeks gestation or ≥400 grams). Of further concern, rates of stillbirth in Australia have remained constant, at approximately 7.4/1000, for over a decade. Late stillbirths are generally defined as those occurring after 28 weeks. The risk of late stillbirth has also remained constant, at approximately 2.9/1000 in Australia and 3.5/1000 in New Zealand.3 Most stillbirths in the developed world occur in the antenatal period and leading contributors include fetal growth restriction, fetal infection, structural or genetic abnormality and maternal medical disease (see Box 1).
A significant proportion of stillbirths are classified as ‘unexplained’, meaning that there is no obvious fetal, placental, maternal or obstetric aetiology, which compounds the frustration and despair felt by families and those caring for them. Important inroads are being made into reducing the number of stillbirths classified as ‘unexplained’ with improvements in investigation, classification and reporting using guidelines such as the Perinatal Society of Australia and New Zealand (PSANZ) Clinical Practice Guideline for Perinatal Mortality (Version 2.2).5
Reducing the risk of stillbirth
Interventions to reduce the global burden of stillbirth have been identified (see Box 2). While many of these interventions are specific to low-income settings, some are equally applicable to high-income settings, where the prevalence of other risk factors is increasing, such as obesity, primiparity, maternal age >35 years and multiple gestation. In such settings, pre-pregnancy weight reduction in obese women, avoidance of delaying childbearing, reducing the risk of iatrogenic multiple pregnancy, smoking cessation and optimising underlying maternal medical conditions should be encouraged. In addition, improved detection and management of fetal growth restriction should be prioritised, which is the focus of this article.
Fetal growth restriction and stillbirth
Fetal growth restriction (FGR) is a leading contributor to stillbirth, with the rate of stillbirth in pregnancies complicated by FGR at least four-times higher than in pregnancies with normal growth.7,8 Importantly, antenatal detection of FGR is associated with a reduction in the risk of stillbirth.7,9 In a cohort of over 92 000 pregnancies, Gardosi et al reported an overall stillbirth rate of 4.2 per 1000, but only 2.4 in pregnancies without FGR. In pregnancies with antentally detected FGR, the stillbirth rate was 9.7 per 1000, increasing to 19.8 per 1000 when it was not detected.7 Detection and management of FGR thus remains one of the leading priorities in the prevention of stillbirth.
Clinical detection of fetal growth and wellbeing
Assessing fetal size
One of the goals of routine antenatal care is identifying poor fetal growth. Abnormal serum analytes from first and second trimester aneuploidy screening, such as low PAPP-A (<0.4 multiple of the median [MoM]), are associated with an increased risk of both FGR and stillbirth, but while they indicate pregnancies that warrant closer surveillance in late pregnancy, their sensitivity and positive predictive value for FGR is low. Clinical detection of fetal size is the mainstay of surveillance for FGR.
Fetal size is most commonly assessed with measurement of the symphysiofundal height (SFH). While SFH measurement reduces inter-observer variability compared to palpation alone, it still only has a sensitivity of 17 per cent and positive predictive value of 20 per cent for the detection of term FGR.10 In addition, the accuracy of SFH may be further compromised in late pregnancy, where the head descends into the pelvis, and in obese women. In an attempt to overcome some of these difficulties, customised fundal height charts have been created, where an optimised fundal height curve is generated, taking into account maternal characteristics. The introduction of customised SFH charts appears to improve detection of FGR.11,12
Assessing fetal wellbeing
Fetal hypoxia as a result of placental insufficiency results in a reduction in non-essential fetal activity and, so, maternal perception of fetal movement is a simple method for monitoring fetal wellbeing. All pregnant women should be advised at each antenatal visit to monitor fetal movements. A report of decreased fetal movements should be investigated with attention to the presence of risk factors for stillbirth, such as FGR, hypertension, diabetes or advanced maternal age; performance of a CTG for fetal wellbeing; and assessment of fetal growth (by abdominal palpation and/or ultrasound). A comprehensive guideline for the management of decreased fetal movements has been developed by the Australian and New Zealand Stillbirth Alliance that provides useful recommendations to assist patients and practitioners.13
Ultrasound detection of fetal growth restriction
Although routine growth scanning has not been shown to be of value in low-risk pregnancies, late pregnancy ultrasound has superior sensitivity for detection of both FGR and macrosomia compared to SFH measurement.14 Standard biometric measures are used in a multiparameter regression equation to derive an estimated fetal weight (EFW) and fetal weight centile for gestational age. The reported centile will depend on which chart is used as the reference range. Traditionally, population or livebirth charts, such as Roberts and Lancaster15, have been used to generate a weight centile, but the limitations of population charts need to be recognised. At preterm gestation, the use of livebirth charts will result in comparing the growth of in utero fetuses to those who have had an indicated, or spontaneous, preterm birth. The risk factors for both spontaneous and indicated preterm birth are associated with important fetal growth decrements (for example, pre-eclampsia and placental abruption) so population charts will always be ‘left skewed’ at preterm gestation, resulting in an under-diagnosis of FGR (EFW<10th centile) and an over-diagnosis of macrosomia (EFW >90th centile).
For this reason, intrauterine growth charts should be used, so that fetal size is being compared to a ‘like’ population of ongoing healthy pregnancies. A further refinement in the use of an intrauterine growth chart is the generation of a customised fetal weight, where the fetal centile is individualised for maternal characteristics, such as height, weight, ethnicity, parity and fetal gender. Customisation is associated with improved detection of FGR and a significant proportion of unexplained stillbirths are found to be growth restricted when customised charts are used.5 Nevertheless, much of this improved sensitivity can be attributed to the use of an intrauterine growth curve alone, with a more modest contribution from the adjustment for maternal characteristics. When a fetus is confirmed to be small, structural, genetic and infective causes should be considered, although the majority of FGR will be ‘deprivational’ in origin, owing to placental insufficiency.

Management of early-onset (<34 weeks) FGR
In the absence of any clearly effective therapy, the mainstay of management following the diagnosis of FGR is close surveillance and timely delivery. In early-onset FGR owing to placental insufficiency, the risks of prematurity need to be weighed against the risks of antenatal hypoxia, acidosis, asphyxial injury and stillbirth. For this reason, once FGR is diagnosed, increased fetal surveillance is required. Integrated fetal testing, involving cardiotocography (CTG), biophysical profile score and arterial and venous fetal Doppler studies, allows the most accurate assessment of fetal wellbeing. Doppler studies of the umbilical artery (UA), middle cerebral artery (MCA) and ductus venosus (DV) have been demonstrated to improve perinatal outcome in high-risk pregnancies, both decreasing obstetric intervention and reducing the risk of perinatal death.16
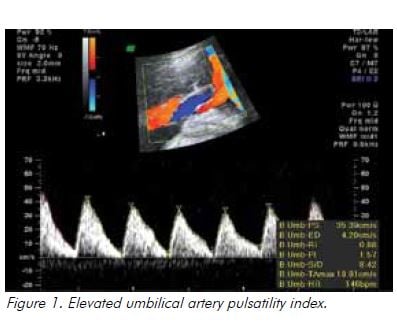
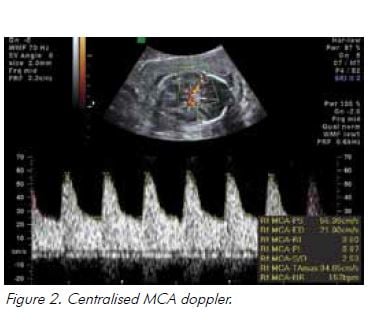
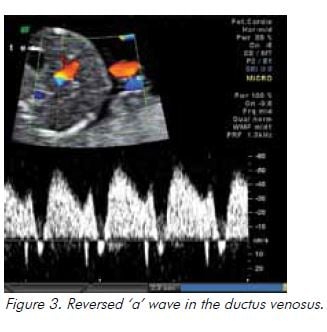
In the setting of hypoxia owing to placental insufficiency, the fetus undergoes a sequence of adaptive behaviours to compensate for reduced oxygen supply.17 Increased resistance to umbilical artery blood flow reflects increasing resistance in the placental bed and is reported as an increased systolic to diastolic (S/D) ratio and/or increased pulsatility index (PI), followed by absent and ultimately reversed end diastolic flow (see Figure 1). Blood is preferentially redistributed toward the fetal brain in an attempt to maintain cerebral oxygenation and maximise survival.18 Dilatation of the fetal cerebral vessels results in an increase in blood flow during diastole and a fall in the MCA-PI (see Figure 2). The lower the MCA-PI, the greater the hypoxia. Amniotic fluid volume decreases when fetal cardiac output is diverted away from the kidneys. Following this, changes in the fetal venous system occur, with a reduced ‘a wave in the DV (see Figure 3). A pulsatile umbilical vein is a preterminal sign.
Early-onset FGR follows a fairly predictable sequence in response to placental insufficiency (as described above) from the arterial to venous circulations, before biophysical abnormalities occur.19 At extremely preterm gestation, advancing gestation is crucial. While the GRIT study found no difference in short- or long-term outcomes according to immediate or deferred delivery for severe preterm FGR20,21, the search for the best ultrasound parameter to use as the final trigger for delivery continues. The TRUFFLE trial randomised 503 women with severe early-onset FGR to delivery according to the results of ductus venosus waveform analysis, or CTG short-term variability monitoring. While the final outcome (infant development at the age of two years) is still pending, preliminary data on the cohort has been published, confirming 92 per cent survival and 70 per cent survival without major morbidity. These outcomes are better than that reported in similar historical cohorts, which suggests outcomes in severe early-onset FGR are optimised when there is a standardised management strategy until 32 weeks.22
Management of late-onset (>34 weeks) FGR
In late-onset FGR, the ultrasound features supporting a diagnosis of placental insufficiency seen at earlier gestation – in particular an abnormal umbilical artery Doppler – are generally absent. Deviations in growth trajectory picked up clinically (with slowing fundal height) may not be detected with ultrasound unless serial assessment of growth has been performed, as is recommended in high-risk pregnancies. This makes the diagnosis of late-onset FGR challenging. In addition, 96 per cent of all births occur after 34 weeks, meaning the burden of FGR in absolute numbers is greatest in the late preterm/term period, with the risk of stillbirth becoming less tolerable as gestation advances and the perinatal risks of prematurity recede.
Fetal doppler may still be useful for surveillance in late-onset FGR, where, among SGA fetuses, centralisation of circulation (evidenced by a low MCA-PI and falling cerebroplacental ratio) is associated with an increased risk of caesarean section, caesarean delivery for fetal compromise and neonatal acidosis.23 In practical terms, fetuses with late-onset FGR should be under close surveillance. In the face of severe FGR (<3rd centile), abnormal uterine artery dopplers or evidence of adaptive fetal behaviours (reducing amniotic fluid, increased cerebral blood flow), delivery should be expedited. In less severe cases, expectant management with close surveillance may be reasonable, although the DIGITAT trial has provided useful evidence that a policy of induction for suspected FGR beyond 36 weeks is not associated with an increase in caesarean section, operative delivery or adverse neonatal outcome.24 The DIGITAT trial did not confirm any reduction in stillbirth, but as it was inadequately powered to do so (with only approximately 320 patients in each arm) induction to minimise the risk of stillbirth remains a reasonable management strategy.
Induction of labour at term to prevent stillbirth
Induction of labour post-term (≥41 weeks) is recognised to be a useful intervention to reduce stillbirth.6 Induction of labour at 41 weeks of gestation results in improved perinatal outcomes without increasing the caesarean section rate.25 The National Institute for Health and Care Excellence (NICE) guideline recommends induction of labour from 41 weeks to prevent late stillbirth.26 In ongoing pregnancies, fetal surveillance (with CTG and AFI) twice weekly after 41 weeks is recommended for post-dates surveillance, with delivery before 42 weeks. The presence of oligohydramnios (AFI <5th centile), which may be associated with an increased risk of adverse outcome, should be a trigger for earlier delivery.27
More controversial is the place of ‘routine’ induction at 39 weeks to minimise the risk of late pregnancy stillbirth. In the last two years, there have been several studies which have suggested – contrary to widespread belief – a policy of liberal induction at 39 weeks does not increase the caesarean section rate.28,29 Indeed, a recent meta-analysis including 31 trials determined that induction of labour was associated with a reduction in the risk of caesarean section (OR 0.83, 95 per cent CI 0.76–0.92)30, with one large study also reporting a significant reduction in perinatal mortality.29 Further study in this area is needed, but these data suggest that routine induction of labour may be able to reduce the risk of stillbirth, without an attendant increase in the risk of operative or caesarean birth.
Management of risk factors other than known FGR
Obesity
Obese women pose a significant challenge, given they are at a significantly increased risk of stillbirth31, although the reasons for this are not completely understood. Given their high rates of co-morbidities, including hypertension and diabetes, and that FGR can be clinically difficult to detect, it is recommended that obese women have an ultrasound evaluation of fetal growth and well-being in late pregnancy.32
History of previous stillbirth
Women with a history of stillbirth have an increased risk of pre-eclampsia (OR 3.1, 95 per cent CI 1.7–5.7), placental abruption (OR 9.4, 95 per cent CI 4.5–19.7), fetal distress (OR 2.8, 95 per cent CI 1.7–4.5), extreme preterm birth (OR 4.2, 95 per cent CI 1.8–9.9), and a 12-fold increased risk of intrapartum stillbirth (95 per cent CI 4.5–33.7), as demonstrated in large retrospective studies.33 Surveillance in the subsequent pregnancy will mostly be dictated by the underlying cause for the previous stillbirth. In women with a history of unexplained stillbirth, ultrasound surveillance of growth in late pregnancy is recommended and increased surveillance, using CTG and/or BPP is often commenced arbitrarily two weeks before the gestation at which the previous stillbirth occurred. Elective delivery is often undertaken by 39 weeks.
Advanced maternal age
Advanced maternal age is independently associated with an increased risk of stillbirth.31,34 Women aged 40 years or older have a similar stillbirth risk at 39 weeks of gestation to 25–29 year olds at 41 weeks of gestation. In view of this, induction of labour at 39 weeks for women of advanced maternal age should be considered to reduce the risk of late pregnancy stillbirths.35
Conclusion
Stillbirth remains a challenge for all maternity care providers in 2013, with the rates of stillbirth remaining static for over a decade. Improved classification systems have reduced the number of unexplained stillbirths and interventions to reduce the global burden of stillbirth have been identified. In high-income countries, obesity, increasing maternal age, primiparity and multiple pregnancy are recognised as potentially modifiable risk factors for stillbirth. Detection of FGR is associated with important reductions in stillbirth rates, owing to the dual interventions of increased surveillance and timely delivery. In women with risk factors other than FGR, increased surveillance of fetal wellbeing in late pregnancy is recommended as well as timely induction at term.
References
- Cousens S, Blencowe H, Stanton C, Chou D, Ahmed S, Steinhardt L, Creanga AA, Tunçalp O, Balsara ZP, Gupta S, Say L, Lawn JE. National, regional, and worldwide estimates of stillbirth rates in 2009 with trends since 1995: a systematic analysis. Lancet. 2011; 377 (9774): 1319.
- Lawn JE, Blencowe H, Pattinson R, Cousens S, Kumar R, Ibiebele I, Gardosi J, Day LT, Stanton C, Lancet’s Stillbirths Series steering committee. Stillbirths: Where? When? Why? How to make the data count? Lancet. 2011; 377(9775): 1448.
- Laws PJ, Li Z, Sullivan EA. Australia’s mothers and babies 2008. Perinatal statistics series Sydney: AIHW National Perinatal Statistics Unit; 2010.
- Chan A, King JF, Flenady V, Haslam RH, Tudehope DI. Classification of perinatal deaths: development of the Australian and New Zealand classifications. J Paediatr Child Health 2004; 40: 340.
- Flenady V, King J, Charles A, Gardener G, Ellwood D, Day K, McCowan L, Kent A, Tudehope D, Richardson R, Conway L, Chan A, Haslam R, Khong Y for the Perinatal Society of Australia and New Zealand (PSANZ) Perinatal Mortality Group. PSANZ Clinical Practice Guideline for Perinatal Mortality. Version 2.2 April 2009.
- Bhutta ZA, Yakoob MY, Lawn JE, Rizvi A, Friberg IK, Weissman E, Buchmann E, Goldenberg RL; Lancet’s Stillbirths Series steering committee. Stillbirths: what difference can we make and at what cost? Lancet. 2011 Apr 30;377(9776):1523-38.
- Gardosi J, Madurasinghe V, Williams M, Malik A, Francis A. Maternal and fetal risk factors for stillbirth: population based study. BMJ. 2013; 346: f108.
- Vashevnik S, Walker S, Permezel M. Stillbirths and neonatal deaths in appropriate, small and large birthweight for gestational age fetuses. ANZJOG 2007 Aug;47(4):302-6.
- Lindqvist PG, Molin J. Does antenatal identification of small-for-gestational age fetuses significantly improve their outcome? Ultrasound Obstet Gynecol 2005; 25: 258–264.
- Sparks TN, Cheng YW, McLaughlin B, Esakoff TF, Caughey AB. Fundal height: a useful screening tool for fetal growth? J Matern Fetal Neonatal Med. 2011 May;24(5):708-12.
- Gardosi J, Francis A. Controlled trial of fundal height measurement plotted on customised antenatal growth charts. Br J Obstet Gynaecol 1999;106: 309-17.
- Roex A, Nikpoor P, van Eerd E, Hodyl N, Dekker G. Serial plotting on customised fundal height charts results in doubling of the antenatal detection of small for gestational age fetuses in nulliparous women. ANZJOG 2012;52: 78-82.
- Preston S, Mahomed K, Chadha Y, Flenady V, Gardener G, MacPhail J, Conway L, Koopmans L, Stacey T, Heazell A, Fretts R and Frøen F for the Australia and New Zealand Stillbirth Alliance (ANZSA). Clinical practice guideline for the management of women who report decreased fetal movements. Brisbane, July 2010.
- Kayem G, Grangé G, Bréart G, Goffinet F. Comparison of fundal height measurement and sonographically measured fetal abdominal circumference in the prediction of high and low birth weight at term. Ultrasound Obstet Gynecol. 2009 Nov;34(5):566-71.
- Roberts CL, Lancaster PA. Australian national birthweight percentiles by gestational age. Med J Aust. 1999 Feb 1;170(3):114-8.
- Alfirevic Z, Stampalija T, Gyte GM. Fetal and umbilical Doppler ultrasound in high-risk pregnancies. Cochrane Database Syst Rev. 2010.
- Ferrazzi E, Bozzo M, Rigano S, Bellotti M, Morabito A, Pardi G, Battaglia FC, Galan HL. Temporal sequence of abnormal Doppler changes in the peripheral and central circulatory systems of the severely growth-restricted fetus. Ultrasound Obstet Gynecol. 2002;19(2):140.
- Schenone MH, Mari G. The MCA Doppler and its role in the evaluation of fetal anemia and fetal growth restriction. Clin Perinatol. 2011 Mar; 38(1):83-102.
- Baschat AA. Fetal growth restriction – from observation to intervention. J Perinat Med. 2010;38(3):239.
- Thornton JG, Hornbuckle J, Vail A, Spiegelhalter DJ, Levene M; GRIT study group. Infant wellbeing at 2 years of age in the Growth Restriction Intervention Trial (GRIT): multicentred randomised controlled trial. Lancet 2004; 364:513–520.
- Walker DM, Marlow N, Upstone L, Gross H, Hornbuckle J, Vail A, Wolke D, Thornton JG. The Growth Restriction Intervention Trial: long term outcomes in a randomized trial of timing of delivery in fetal growth restriction. Am J Obstet Gynecol 2011; 204: 34.e1–9.
- Lees C, Marlow N, Arabin B et al and the TRUFFLE Group (2013). Perinatal morbidity and mortality in early-onset fetal growth restriction: cohort outcomes of the trial of randomized umbilical and fetal flow in Europe (TRUFFLE). Ultrasound Obstet Gynecol, 42: 400–408. doi: 10.1002/uog.13190.
- Cruz-Martínez R, Figueras F, Hernandez-Andrade E, Oros D, Gratacos E. Fetal brain Doppler to predict cesarean delivery for nonreassuring fetal status in term small-for-gestational-age fetuses. Obstet Gynecol. 2011 Mar;117(3):618-26.
- Boers KE, Vijgen SMC, Bijlenga D et al on behalf of the DIGITAT study Group. Induction versus expectant monitoring for intrauterine growth restriction at term: randomised equivalence trial (DIGITAT). BMJ 2010; 341:c7087.
- Gülmezoglu AM, Crowther CA, Middleton P. Induction of labour for improving birth outcomes for women at or beyond term. Cochrane Database Syst Rev 2006;(4):CD004945.
- National Institute for Health and Clinical Excellence. Induction of Labour. London; NICE:2008.
- Morris JM, Thompson K, Smithey J, Gaffney G, Cooke I, Chamberlain P, Hope P, Altman D, MacKenzie IZ. The usefulness of ultrasound assessment of amniotic fluid in predicting adverse outcome in prolonged pregnancy: a prospective blinded observational study. Br J Obstet Gynaecol 2003;110(11):989.
- Darney BG, Snowden JM, Cheng YW, et al. Elective Induction of Labor at Term Compared With Expectant Management: Maternal and Neonatal Outcomes. Obstet Gynecol 2013; 122:761.
- Stock SJ, Ferguson E, Duffy A, Ford I, Chalmers J, Norman JE. Outcomes of elective induction of labour at term compared with expectant management: population based study. BMJ 2012 May;344:e2838.
- Wood S, Cooper S, Ross S. Does induction of labour increase the risk of caesarean section? A systematic review and meta-analysis of trials in women with intact membranes. BJOG 2013; DOI: 10.1111/1471-0528.12328.
- Flenady V, Koopmans L, Middleton P, Frøen JF, Smith GC, Gibbons K, et al. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet 2011;377:1331–40.
- Consultative Council on Obstetric and Paediatric Mortality and Morbidity (CCOPMM). Annual report for the year 2007. Available at: ww.health.vic.gov.au/ccopmm/OPMM.
- Sharma PP, Salihu HM, Kirby RS. Stillbirth recurrence in a population of relatively low-risk mothers. Paediatr Perinat Epidemiol 2007;21 Suppl 1:24–30.
- Reddy UM, Ko CW, Willinger M. Maternal age and the risk of stillbirth throughout pregnancy in the United States. Am J Obstet Gynecol 2006;195:764–70.
- Dhanjal MK and A Kenyon for the Scientific Advisory Committee of the RCOG. Induction of Labour in Older Mothers. Scientific Impact Paper No.34. RCOG. February 2013.






Leave a Reply