Cancer treatments have improved dramatically over the past few decades, such that the majority of young women diagnosed with the commonest forms of cancer can expect to be cured.1 The concern of treating physicians now is not just provision of a ‘disease-free state’, but also the preservation of an optimum ‘quality of life’ following chemotherapy treatment.
This has resulted, fortunately, in greater awareness and recognition of the importance of the long-term effects of cancer treatment.
Rising cancer survival rates have also led to an increased interest in trying to reduce the risk of premature menopause in affected patients, without compromising the efficacy of chemotherapy.2,3 In particular, it is recognised that certain chemotherapy treatment regimens, especially those including high-dose alkylating agents, can lead to reduced fertility. This is a serious side effect for young women who would otherwise expect to lead a normal life after surviving cancer, including raising a family of their own.4
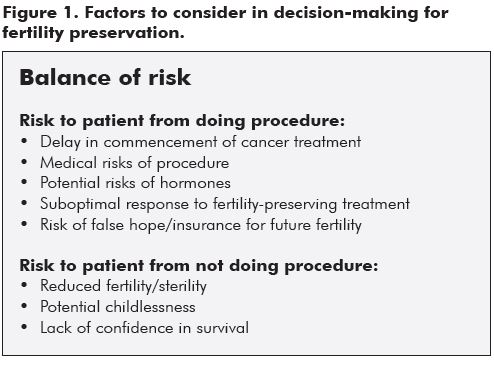
The diagnosis of a potentially life-threatening malignancy, requiring toxic chemotherapy, is an extremely traumatic and shattering experience for young women and their families. Discussion of further evaluation, therapeutic choices and the short and longer term implications of these, as well as discussion of prognosis, is extremely challenging for oncologists and their teams. Figure 1 lists some of the issues which need to be considered when making decisions about fertility preservation. Counselling often requires several consultations, despite the pressure of time for commencement of treatment. Incorporation of discussion about threats to future fertility and options to preserve and protect fertility, can potentially add an extra layer of complexity and often confusion to an already fraught interaction. However, patients and their families invariably welcome the information imparted regarding future fertility. This information, and perhaps most importantly, the referral for fertility discussion, can give young women and their families optimism about future survival and quality of life. It can also provide an opportunity for them to feel more in control of their situation and to make choices which suit their individual life situation. Counselling is of paramount importance, as many young people will choose not to avail themselves of any interventionist options, but will appreciate being informed of both the implications of their cancer and its treatment on future fertility and the choices available. This helps them to feel more in control of their reproductive future. For those who wish to be more active regarding future fertility, we can now offer the various options with increasing confidence and optimism.
How big is the problem? Cancer risk for young women in Australia
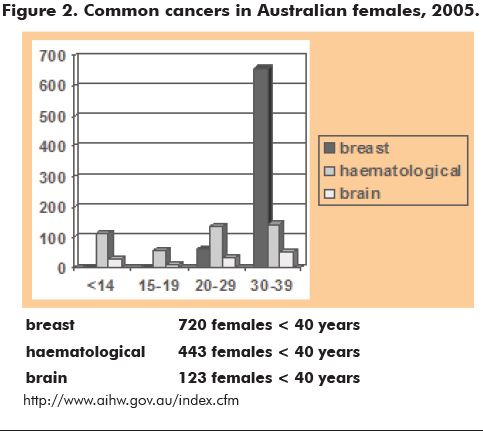
Cancer prevalence increases with age, but data from the Australian Institute for Health and Welfare (AIHW) suggest that the risk of cancer in young women is not insignificant. Over 770 women less than 40 years of age were diagnosed with cancer in Victoria alone in 2004 (CCV 2005). Although survival and prognoses are gradually improving, the incidence of cancer is not diminishing. In 2010, it is expected that over 70/100,000 girls less than 20 years of age and over 380/100,000 young women aged 20 to 39 years will be diagnosed with cancer (www.aihw.gov.au/publications/can/cipa02-11/cipa02-11.pdf). Figure 2 shows the incidence of the common cancers in young Australian women in 2005.
Risk of damage from cancer treatment
Chemotherapy is commonly associated with a temporary cessation of menstrual and ovulatory function, with hot flushes and amenorrhoea.5,6,7 This may persist into more ‘permanent’ ovarian failure and premature menopause, or may resolve over six to 18 months and thus be diagnosed as ‘temporary’ only in retrospect. Sometimes ovarian function may appear to persist normally, as evaluated by clinical or hormonal measures.
Although many young women continue to menstruate regularly after chemotherapy, or resume menses after a period of amenorrhoea, morphological and ultrasound assessments usually demonstrate reduced follicle numbers, and endocrine assessment commonly reveals alterations in levels of follicle-stimulating hormone (FSH), anti-Mullerian hormone (AMH), inhibin B (InhB) and luteinising hormone (LH), indicating persistent vulnerability to premature ovarian failure.8,9 Thus, resumption of ‘normal’ menstrual cycles after cancer treatment may unwittingly, but inaccurately, reassure physicians and their patients, with potentially serious long-term fertility consequences.
Many anti-cancer drugs exert their actions predominantly on dividing cells. The toxic effects of chemotherapy treatments may include inhibition of cell division and adverse effects on DNA function within the dividing granulosa and theca cells of the ovary, as well as the oocytes contained within the follicle. It has been confirmed that exposure to various alkylating agents during chemotherapy treatment (in particular cyclophosphamide and procarbazine), with resultant effects on the ovaries, are age-dependent.7 As the number of oocytes declines with advancing age, the ovaries of older individuals become more vulnerable to gonadal toxins relative to the ovaries of younger women and girls. An individual young woman’s chance of developing both acute and permanent ovarian failure is related to increasing age, diagnosis and the specific treatment modalities used. Radiotherapy and chemotherapy, with the use of alkylating agents in particular, all increase risk.7,10 Radiotherapy to the pelvis can also cause serious damage to the uterus, particularly the endometrium and the myometrium. Cranial radiation may affect pituitary hormone production and release.
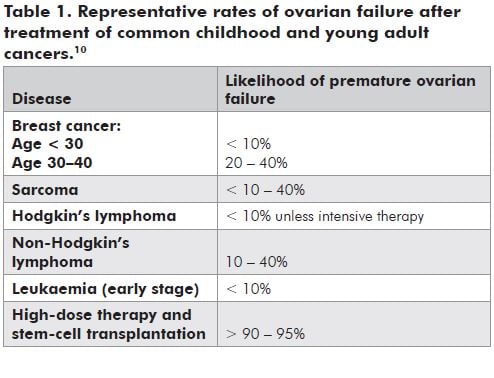
Table 1 illustrates the range of fertility risks associated with treatment of the commonest cancers.10 However, given the scarcity of data regarding rates of infertility following most cancer treatments, oncologists may have difficulty in providing specific and accurate data to patients about their particular risks for infertility.
Options for fertility preservation and protection
Given the potentially toxic effects of chemotherapy agents on the ovary, with the resultant risk of temporary and more permanent ovarian failure, the availability of options to preserve and protect fertility is of great importance to these young women and their families. Current options to preserve fertility in this patient population of women who undergo chemotherapy treatment are limited, but include preservation of embryos, oocytes or ovarian tissue prior to cancer treatment, and ovarian protection with the use of gonadotrophin-releasing hormone (GnRH) analogues throughout the duration of treatment.11
Protection of the ovaries during chemotherapy
GnRH analogues may be agonists or antagonists, inducing their effects by either down-regulation or competitive inhibition respectively. In women, they induce a temporary and reversible medical state of hypo-oestrogenism by decreasing pituitary FSH and LH. GnRH analogues are commonly used in the treatment of endometriosis, in some infertility treatments to prevent the LH surge, and as adjuncts to chemotherapy in hormonally sensitive breast and prostate cancers. In children, they have also been utilised in the treatment of central precocious puberty.
Recently, GnRH analogues have been shown to protect the ovary from damage during chemotherapy in some animal models.12,13 The mechanism of this protective effect is poorly understood, particularly as the early phases of follicle development are thought to be gonadotrophin-independent.14 Possibly, the protective effect could be mediated either by reduction of blood flow to the ovary or through modulation of AMH activity.
Most human studies of GnRH analogues to date involving cancer patients have been uncontrolled and/or retrospective. There is thus little prospective data specifically addressing the issues for those women of reproductive age who are at highest risk of infertility from chemotherapy. However, despite the above limitations, there is now increasing evidence from clinical studies to suggest a therapeutic benefit from GnRH agonists for ovarian protection.
The recent publication of a randomised trial15, together with several reviews16,17, provide support for the role of GnRH analogues for ovarian protection. They also lay the ground-work for further prospective studies. Other medications, including immunomodulators, are also being trialled as ovarian protectors in animal models. It is important to note, however, the ‘chemoprotection’ of the ovaries should still be considered experimental. Patients should be counselled accordingly, especially about the lack of large studies currently available, and where possible they should be enrolled in clinical trials.
Embryo freezing
Cryopreservation of embryos in an IVF cycle prior to the onset of cytotoxic treatment offers the best chance of a subsequent pregnancy, should a woman subsequently become infertile after chemotherapy. This is an established technology whereby the ovaries are stimulated with gonadotrophins to produce mature oocytes. These are then harvested and fertilised with the partner’s (or, occasionally, donor) sperm. Resultant embryos are then frozen and stored until required. The survival rate of embryos after freezing and thawing is in the range of 75 to 90 per cent, and implantation rates (clinical pregnancy rate for each individual embryo transferred) are between 18 and 30 per cent (currently 25 to 30 per cent in women under 37 years of age), approximating spontaneous fertility.18 If multiple embryos are available, the cumulative pregnancy rate can be more than 60 per cent.
Unfortunately, there are sometimes barriers to the use of this technique in the oncology setting. Often, commencement of chemotherapy is required forthwith after diagnosis, so there is not enough time for the ten to 16 days required to initiate and complete an IVF cycle. Additionally, younger women or adolescents may not have a stable partner and for young women it may not be appropriate to consider donor sperm. In some jurisdictions, if a relationship subsequently dissolves, the woman may not be able to utilise the embryos if her ex-partner refuses to give permission. In addition, many women with a malignancy do not respond well to standard ovarian stimulation regimens, perhaps because of intense physical and psychological stress associated with the cancer diagnosis, such that the numbers and/or quality of oocytes obtained may not be optimal. Finally, there are theoretical concerns that the endocrine stimulation required to produce suitable oocytes may have an adverse effect on hormonally sensitive tumours such as breast or endometrial cancer.7
Oocyte freezing
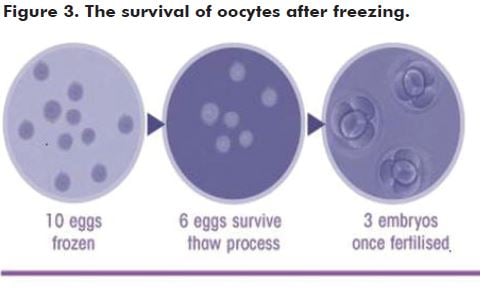
An alternative to embryo freezing is to freeze mature oocytes after ovarian stimulation. This maintains autonomy, as there is no requirement for a partner or sperm donor. Freezing of mature oocytes has been practised for well over a decade19, but for many years the fragility of the oocyte, compared with the embryo, hampered success rates with viability after thawing. Recent improvements in freeze-thaw protocols, however, make this a much more reliable option with reports of over 60 per cent of mature oocytes surviving the thaw, and subsequent fertilisation rates now approximate those for fresh oocytes during IVF.19
Over 930 births have now been reported from oocyte freezing (including several in Australia), with no major increase in complication rates such as miscarriage or congenital abnormalities.20 Like embryo freezing, however, the requirement for hormonal stimulation and time may preclude the use of oocyte freezing for some young women about to embark on chemotherapy.
Ovarian tissue freezing and grafting
In some centres, patients may be offered the opportunity to harvest and freeze ovarian tissue prior to the commencement of cancer treatment. The tissue is obtained laparoscopically, with the surgeon usually taking up to one-third of one ovary, or a whole ovary if high-dose chemotherapy or pelvic radiotherapy is planned for cancer treatment.
This option has an advantage in that there is no requirement for stimulation, meaning that it can be undertaken rapidly. However, it exposes the patient to the risk of laparoscopy, which carries approximately a 0.5 to two per cent chance of conversion to open laparotomy and a one in 12,000 chance of death.21 At the time
of the European Society for Human Reproduction and Embryology (ESHRE) meeting in Rome this year, there have only been 14 live births reported worldwide following the re-implantation of thawed ovarian tissue. Follicular development from grafted ovarian tissue does not always follow typical cyclical patterns, making spontaneous fertility and IVF more difficult than in the conventional setting.22 Although the harvested tissue is rigorously and repeatedly tested by histological and other immunohistochemical and molecular techniques, concerns have been expressed that small ovarian vessels, or even the ovarian tissue itself, has the potential to harbour malignant cells, particularly in acute leukaemia.23
It is hoped that further improvements in freeze-thaw technology may allow frozen ovarian tissue to be a more dependable form of fertility preservation in the near future. This field is rapidly evolving, so patients should be counselled that while this technique is currently highly experimental, there is the potential to preserve a large number of follicles within the tissue, and the results to date are very encouraging. Again, this procedure should only be performed in the setting of a properly-conducted clinical trial.
Conclusion
It is now acknowledged that discussion of future fertility options should be considered an essential part of the treatment plan for young women having gonadotoxic therapy. Increasing research and clinical collaboration and cooperation between reproductive medicine specialists and oncological teams will allow us to provide our patients with information which can help them to have more control over their reproductive future.
References
- Jemal A, Murray T, Samuels A, Ghafoor A, Ward E and Thun MJ. Cancer Statistics, 2003. CA Cancer J Clin. 2003; 53: 5-26.
- Chen WY, Manson JE. Premature ovarian failure in cancer survivors: new insights, looming concerns. J Natl Cancer Inst. 2006; 98: 880-881.
- Royal College of Physicians, Royal College of Radiologists, Royal College of Obstetricians and Gynaecologists. The effects of cancer treatment on reproductive functions: Guidance on management. Report of a Working Party. London: RCP, 2007.
- Schover LR. The cancer experience and motivation for biological and social parenthood. Parenthood after Cancer Conference. Texas: MD Anderson Cancer Center, 2004.
- Warne GT, Fairley KF, Hobbs JB, Martin FI. Cyclophosphamide induced ovarian failure. N Eng J Med. 1973: 289-1159.
- Nicosia SV, Matus-Ridley M, Meadows AT. Gonadal effects of cancer therapy in girls. Cancer. 1985; 55: 2364.
- Meirow D. Ovarian injury and modern options to preserve fertility in female cancer patients treated with high dose radio-chemotherapy for hemato-oncological neoplasias and other cancers. Leuk Lymphoma. 1999; 33: 65-76.
- Anderson RA, Themmen AP, Al-Qahtani A, Groome NP and Cameron DA. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod. 2006; 21: 2583-92.
- Sklar CA. Reproductive physiology and treatment related loss of sex hormone production. Med Pediatr Oncol. 1999; 33: 2-8.
- Stern CJ, Toledo MG, Gook DA, Seymour JF. Fertility preservation in female oncology patients. ANZJOG 2006; 46: 15-23.
- Levine J, Canada A, Stern CJ. Fertility reservation in adolescents and young adults with cancer. Early release, published online May 10 2010. J Clin Oncol. 10.1200/JCO.2009.22.8312.
- Ataya K, Rao LV, Lawrence E, Kimmel R. Luteinising hormone-releasing hormone agonist inhibits cyclophosphamide-induced ovarian follicular depletion in rhesus monkeys. Biol Reprod. 1995; 52: 365-372.
- Meirow D, Assad G, Dor J, Rabinovici J. The GnRH antagonist cetrorelix reduces cyclophosphamide-induced ovarian follicular destruction in mice. Hum Reprod. 2004; 19: 1294-1299.
- McNatty KP, Smith P, Moore LG, Reader K, Lun S, Hanrahan JP, et al. Oocyte-expressed genes affecting ovulation rate. Mol Cell Endocrinol. 2005; 234: 57-66.
- Badawy A, Elnashar A, El-Ashry M, Shahat M. Gonadotropin-releasing hormone agonists for prevention of chemotherapy-induced ovarian damage: Prospective randomized study. Fertil Steril. 2009; 91: 694-697.
- Beck-Fruchter R, Weiss A, Shalev E. GnRH agonist therapy as ovarian protectants in female patients undergoing chemotherapy: a review of the clinical data. Hum Reprod update 2008; 14: 553-561.
- Blumenfeld Z, von Wolff M. GnRH-analogues and oral contraceptives for fertility preservation in women during chemotherapy. Hum Reprod update 2008; 14: 543-552.
- Jansen RP. The effect of female age on the likelihood of a live birth from one in-vitro fertilisation treatment. Med J Aust. 2003; 178: 258-261.
- Gook DA, Edgar DH. Human oocyte cryopreservation. Hum Reprod update 2007; 13: 591-605.
- Noyes N, Porcu E, Borini A. Over 900 oocyte cryopreservation babies born with no apparent increase in congenital anomalies. Reprod Biomed Online 2009; 18: 769-776.
- Jansen FW, Kapiteyn K, Trimbos-Kemper T, Hermans J, Trimbos JB. Complications of laparoscopy: a prospective multicentre observational study. Br J Obstet Gynaecol. 1997; 104: 595-600.
- Demeestere I, Simon P, Emiliani S, Delbaere A and Englert Y. Orthotopic and heterotopic ovarian tissue transplantation. Hum Reprod update 2009; 15: 649-665.
- Seshadri T, Gook D, Lade S, Spencer A, Grigg A, Tiedemann K, et al. Lack of evidence of disease contamination in ovarian tissue harvested for cryopreservation from patients with Hodgkin lymphoma and analysis of factors predictive of oocyte yield. Br J Cancer. 2006; 94: 1007-1010.






Leave a Reply